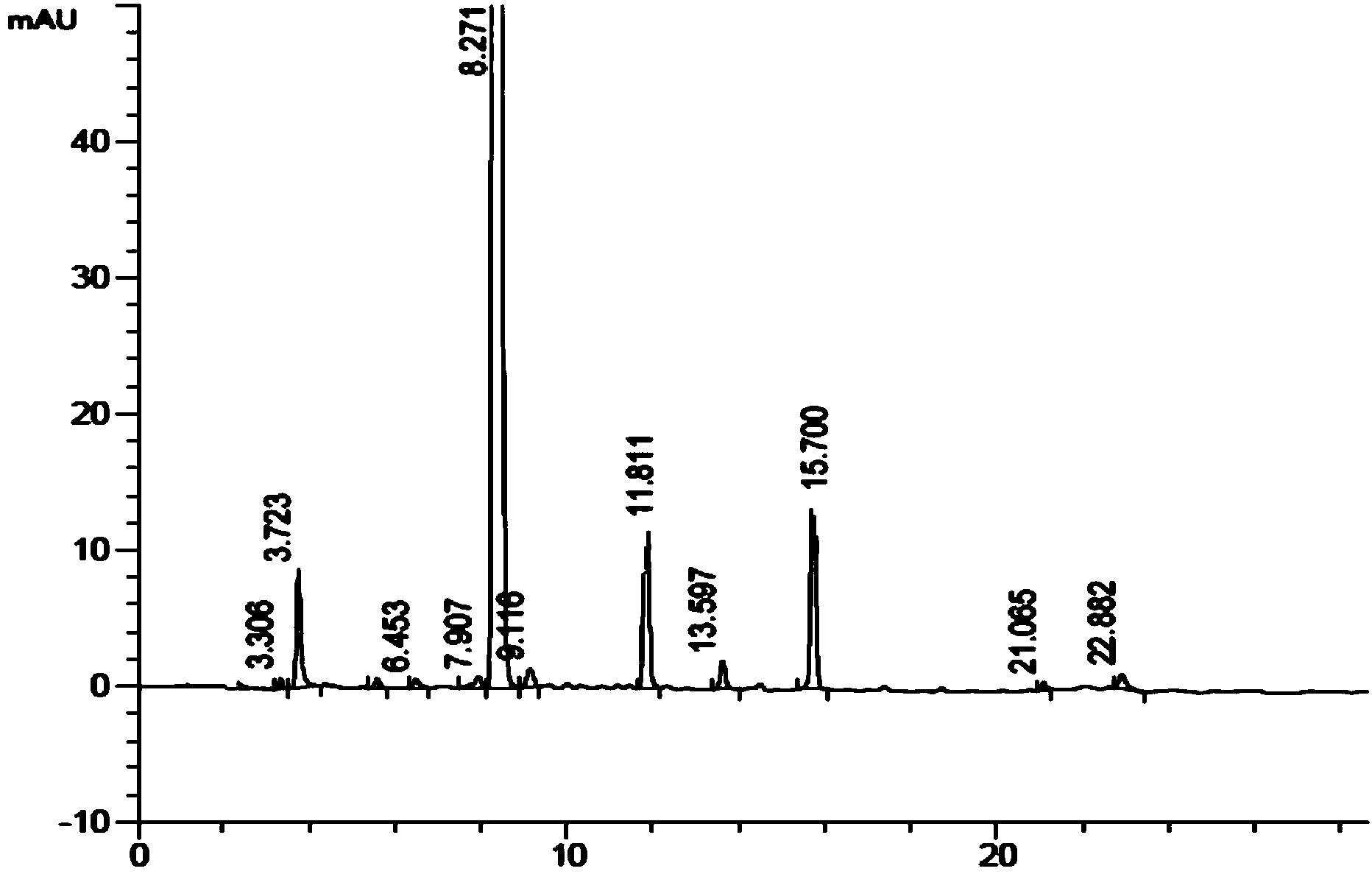
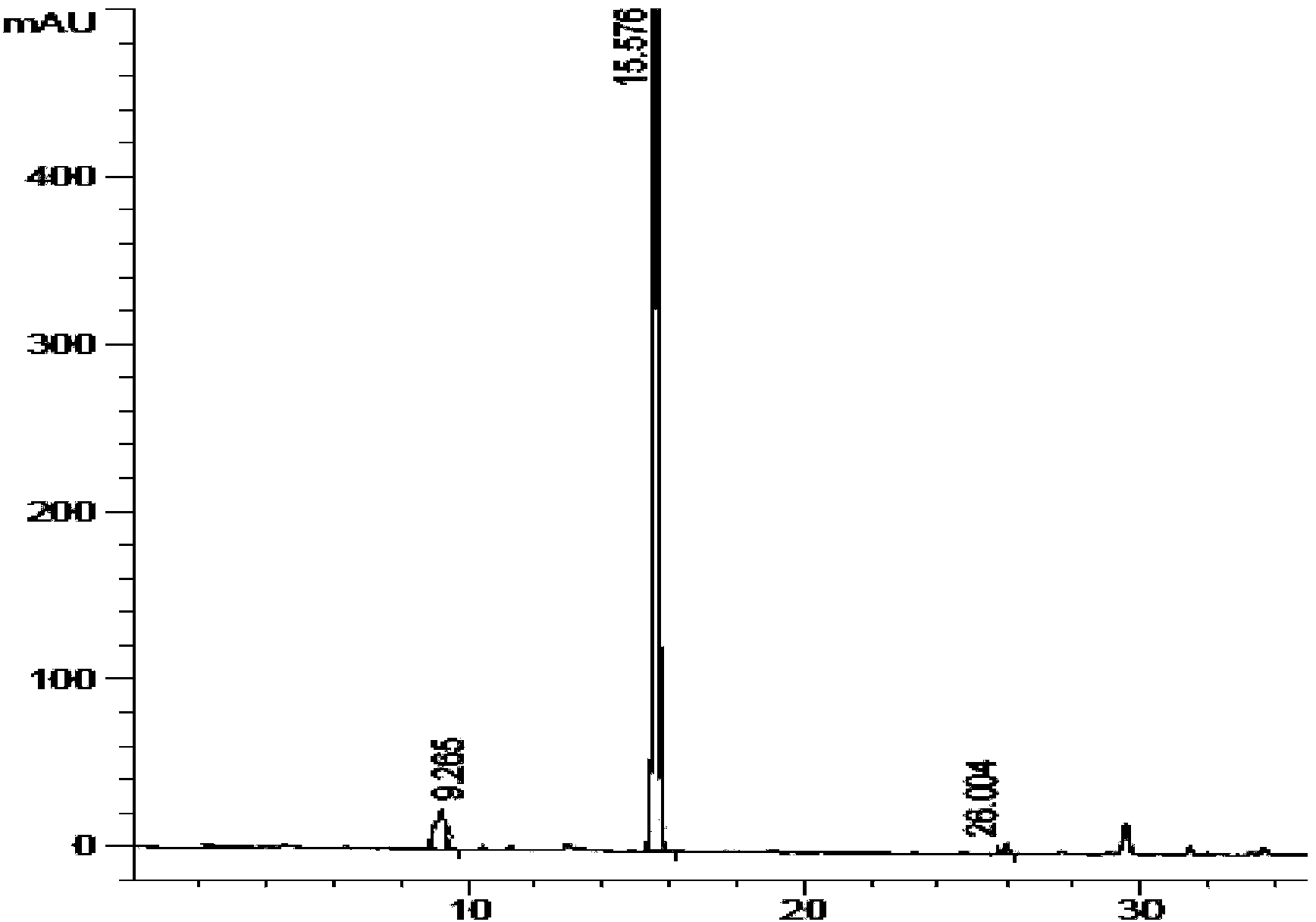
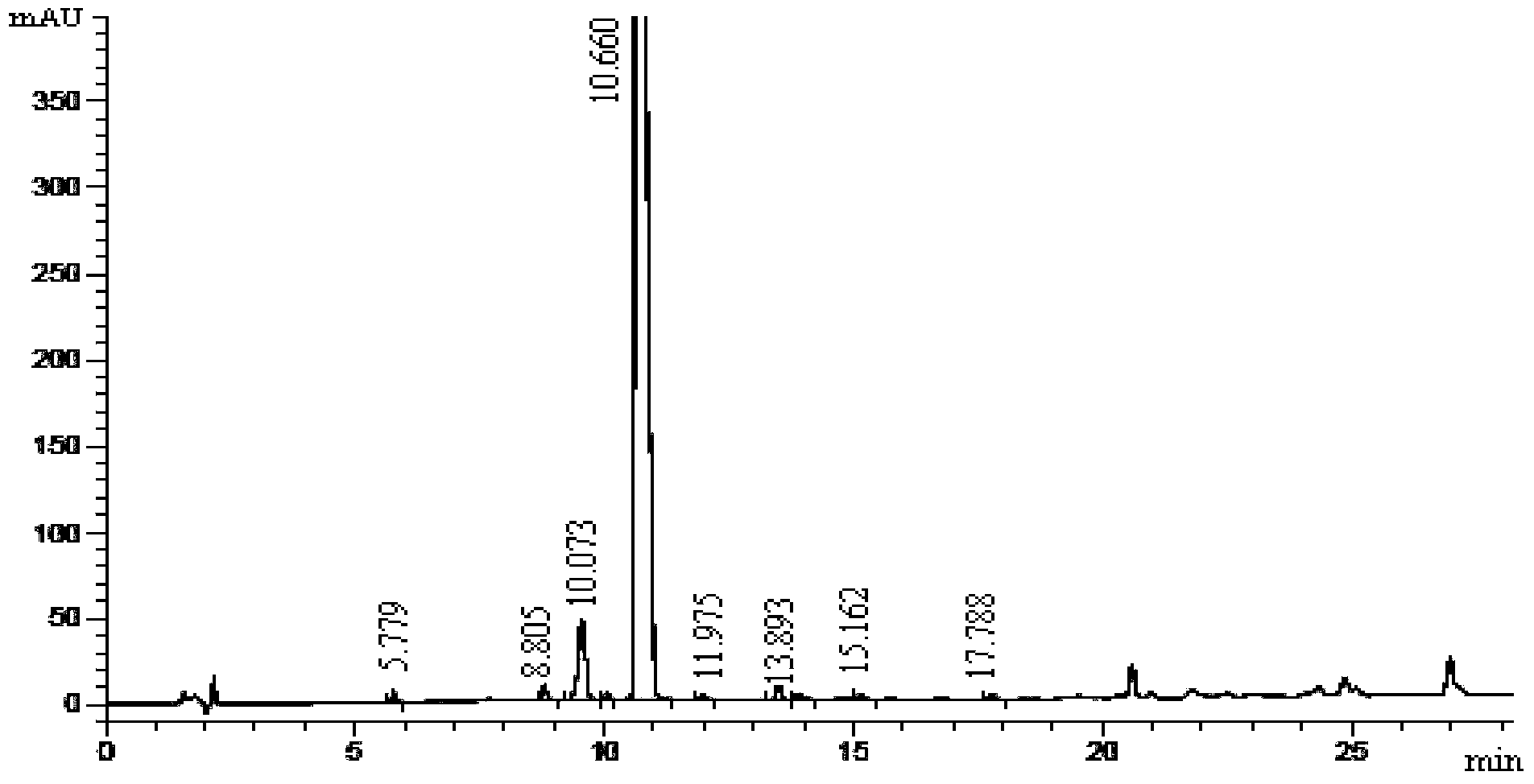
Carfilzomib intermediate and preparation method thereof, as well as preparation method of carfilzomib
A technology for carfilzomib and intermediates, applied in the field of preparation of carfilzomib, carfilzomib intermediates and its preparation, can solve the problem of low final product yield, multiple starting materials, low reaction yield, etc. problem, to achieve the effect of improving yield and purity, mild and controllable reaction conditions, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The preparation process of the intermediate is simple, only involves condensation and deprotection reactions between amino acids, and the reaction conditions are mild and controllable. To prepare carfilzomib using the intermediate as a raw material, only the epoxidation reaction can be carried out, and the epoxidation reaction conditions adopted have higher selectivity, which improves the yield and purity of carfilzomib, and prepares The yield of carfilzomib is more than 35%, and the purity is more than 99%, which provides an economical and environment-friendly synthesis process for the preparation of carfilzomib.
[0046] A carfilzomib intermediate provided by the present invention has a structure represented by formula (XIII).
[0047] The present invention also provides a method for preparing the above-mentioned carfilzomib intermediate, comprising:
[0048] Under the action of a condensing agent and an organic base, the compound shown in the formula (XI) is condens...
Embodiment 1
[0103] Preparation of compound shown in embodiment 1 formula (XII)
[0104] Add 23.1g (0.10mol) of N-Boc-L-leucine with the structure shown in formula (1) into a 1000mL three-necked flask, add 400mL of dichloromethane, stir and dissolve; cool the solution to -10°C under stirring, and pour 78.1 g (0.15 mol) of PyBOP was added thereto, and a dichloromethane (100 mL) solution of 10.7 g (0.11 mol) of N,O-dimethylhydroxylamine hydrochloride was slowly added dropwise to the mixture. The resulting solution was stirred at room temperature for 10 h, then diluted with water, extracted twice with dichloromethane, combined organic phases, washed with saturated brine, dried over anhydrous magnesium sulfate, and filtered to remove anhydrous magnesium sulfate. The solvent was evaporated to dryness under reduced pressure to obtain 23.6 g of the compound represented by formula (2).
[0105] Under the protection of nitrogen, add 13.7g (0.05mol) of the compound represented by the formula (2) in...
Embodiment 2
[0107] Preparation of compound shown in embodiment 2 formula (V-a)
[0108] Under nitrogen protection, add 27.9g (0.10mol) of N-Boc-L-homophenylalanine and 26.0g (0.105mol) of L-leucine methyl ester trifluoroacetate into a 1000mL three-necked flask, and add 500mL Tetrahydrofuran, and 51.7 g (0.4 mol) of DIEA were added thereto, and the mixture was cooled to 0° C. with stirring. To this mixture was added HOBT 14.9 g (0.11 mol). The reactant was placed under nitrogen, stirred for 2 h, distilled under reduced pressure, the residue was dissolved in 300 mL of dichloromethane, and washed twice with saturated sodium bicarbonate, water and saturated saline, each time 150 mL , the organic layer was evaporated to dryness under reduced pressure to obtain 36.5 g of the compound represented by formula (IV), and then 120 mL of a mixed solution of trifluoroacetic acid:dichloromethane=4:1 was added thereto, stirred and reacted at 15° C. for 7 h, and concentrated to obtain off-white Solid 29...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com