Preparation method of active targeting adriamycin amycin-amphipathic chondroitin sulfate micelle
A chondroitin sulfate and amphiphilic technology, which is applied in the field of active targeting doxorubicin-amphiphilic chondroitin sulfate micelles, can solve the problem of lack of targeted tumor cell drug resistance and lack of target for anti-tumor drugs. Tropism, lack of targeting of tumor cells, etc., to achieve good application prospects, easy to pass through, and less interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
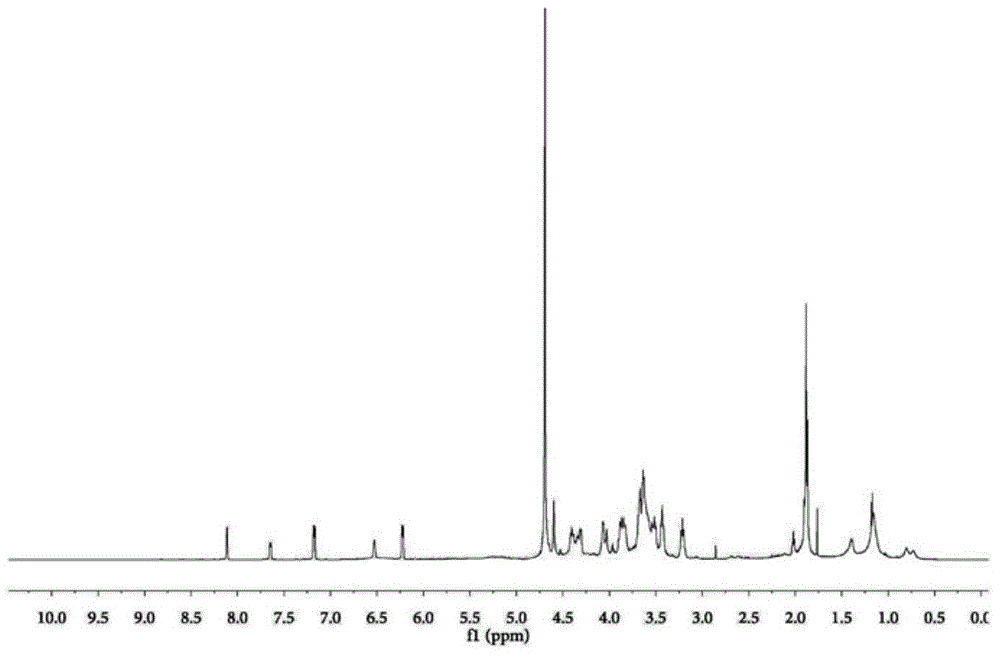
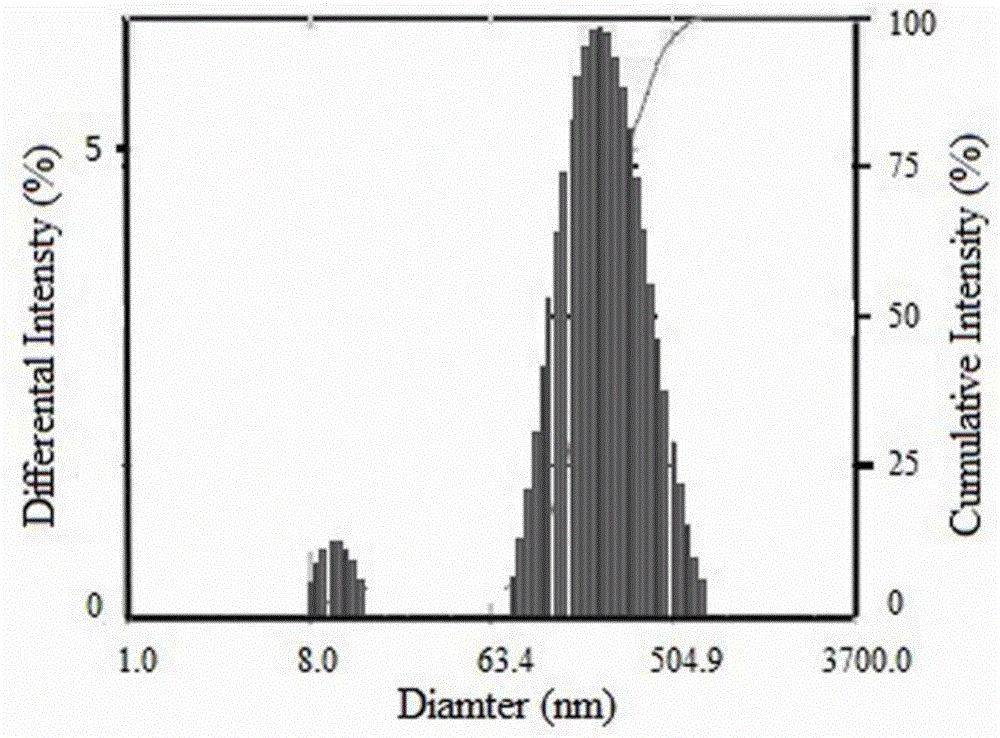
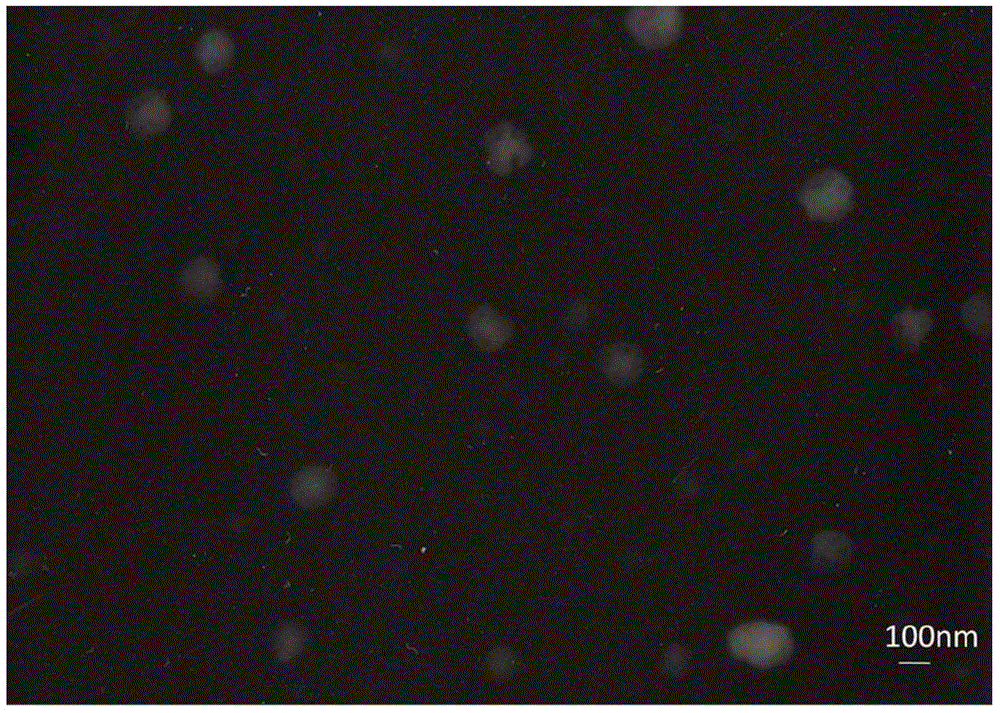
[0045] Example 1 Synthesis of folic acid-linoleoyl-shark chondroitin sulfate and preparation of doxorubicin nanoparticles
[0046] 1. Synthesis of Linoleoyl-Shark Chondroitin Sulfate
[0047] 5g shark chondroitin sulfate ( M w: 20,000), 0.5g DMAP, 100mL formamide, stir and dissolve at 55°C, add 1.5g of linoleoyl chloride for esterification reaction, stir at 55°C for 1h, dialyze the reaction solution with deionized water for 48h (change the water once every 6h) , to remove DMAP and formamide. Continue dialysis in 95% ethanol solution for 48h to remove free linoleic acid. The yellowish product obtained after dialysis was washed twice with 5 mL of 95% ethanol, and dried in vacuo at 45°C. The obtained yellowish solid powder was linoleoyl-sarcochondroitin sulfate, and the substitution degree of linoleic acid was 4.8%.
[0048] 2. Synthesis of Folate-Linoleoyl-Shark Chondroitin Sulfate
[0049] Add 0.2g folic acid to 2mL DMSO, add 3 drops of triethylamine dropwise, stir to dis...
Embodiment 2
[0052] Example 2 Synthesis of folic acid-linoleoyl-low molecular weight porcine chondroitin sulfate and preparation of doxorubicin nanoparticles
[0053] 1. Synthesis of Linoleoyl-Low Molecular Weight Porcine Chondroitin Sulfate
[0054] 10g low molecular weight porcine chondroitin sulfate ( M w: 4,000), 1g DMAP, 100mL formamide, stir and dissolve at 55°C, add 3g of linoleoyl chloride for esterification reaction, stir at 55°C for 1h, dialyze the reaction solution with deionized water for 48h (change the water every 6h), remove DMAP and formamide. Continue dialysis in 95% ethanol solution for 48h to remove free linoleic acid. The yellowish product obtained after dialysis was washed twice with 5 mL of 95% ethanol, and dried in vacuum at 45°C. The obtained yellowish solid powder was linoleoyl-low molecular weight porcine sulfate, and the substitution degree of linoleic acid was 5.3%. .
[0055] 2. Synthesis of Folate-Linoleoyl-Low Molecular Weight Porcine Chondroitin Sulfat...
Embodiment 3
[0059] Example 3 Synthesis of folic acid-α-linolenoyl-low molecular weight porcine chondroitin sulfate and preparation of doxorubicin nanoparticles
[0060] 1. Synthesis of α-linolenoyl-low molecular weight porcine chondroitin sulfate
[0061] 5g low molecular weight porcine chondroitin sulfate ( M w: 4,000), 0.5g DMAP, 100mL formamide, stir and dissolve at 55°C, add 1.5g of α-linolenoyl chloride for esterification reaction, stir at 55°C for 1h, dialyze the reaction solution with deionized water for 48h (change the water once every 6h ), remove DMAP and formamide. Continue dialysis in 95% ethanol solution for 48h to remove free α-linolenic acid. The yellowish product obtained after dialysis was washed twice with 5 mL of 95% ethanol, and dried in vacuum at 45°C. The resulting yellowish solid powder was α-linolenoyl-low molecular weight porcine chondroitin sulfate. 5.2%.
[0062] 2. Synthesis of Folate-α-Linolenoyl-Low Molecular Weight Porcine Chondroitin Sulfate
[0063]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com