A drug betaine conjugate, its pharmaceutical composition and application
A technology of conjugates and betaine, which is applied in the field of medicine, can solve the problems of complex synthesis routes of drug phospholipid compounds, drug leakage, and difficulty in drug efficacy, and achieve low toxicity drug efficacy or anti-tumor activity, low toxicity Effects of side effects, superior efficacy, or antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
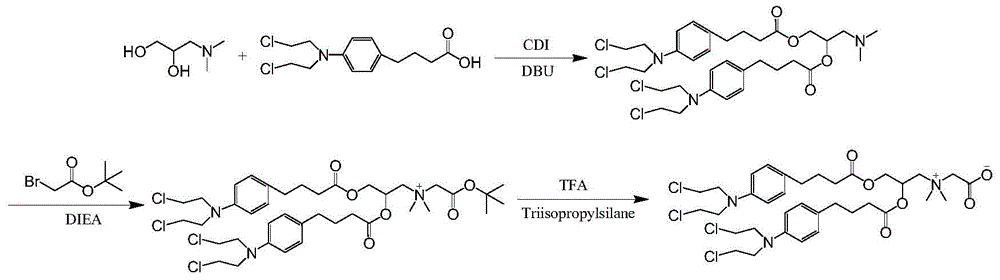
[0095] Synthesis of chlorambucil betaine conjugates (synthetic route see figure 1 )
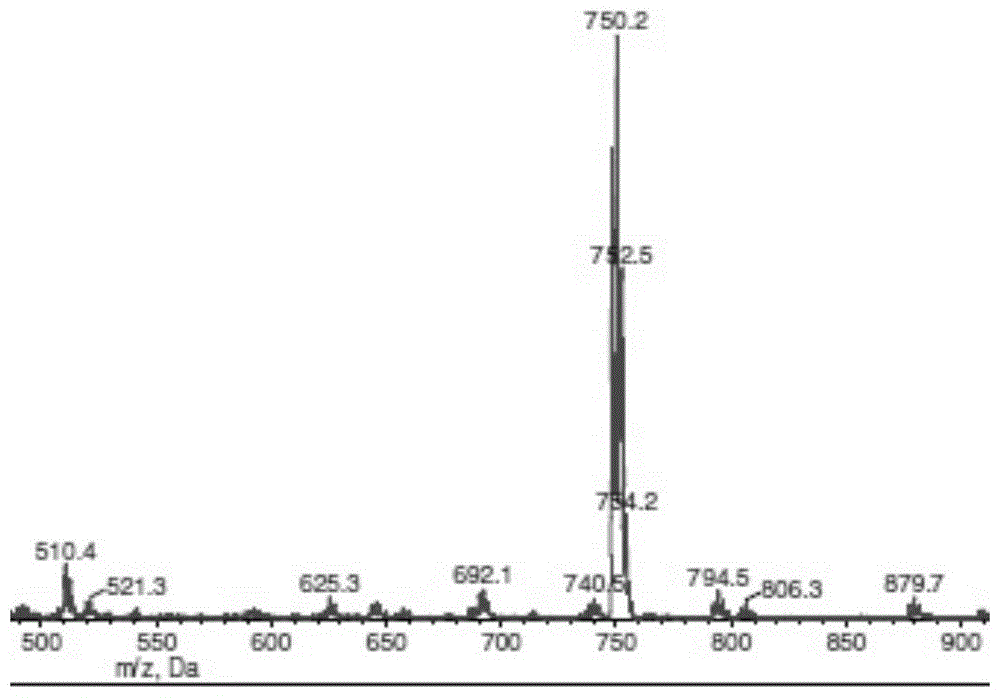
[0096] Chlorambucil (1.818g, 6mmol) and CDI (1.236g, 6mmol) were dissolved in 20mL of dichloromethane, stirred at 25°C for 2h, and filtered. 3-Dimethylamino-1,2-propanediol (0.119g, 1mmol) and DBU (0.244g, 0.2mmol) were added to the filtrate, and heated under reflux for 24h. The reaction solution was diluted to 50mL with dichloromethane, washed 3 times with 1M hydrochloric acid, Na 2 SO 4 After drying, the solvent was evaporated to dryness to obtain product 1. The reaction process was detected by TLC (the developing solvent was methanol:dichloromethane / 1:1, and ultraviolet detection (λ=254nm)); the structure of the product was determined by mass spectrometry. MS:[M+H] + m / z 690.4.
[0097] Dissolve product 1 (0.692g, 1mmol) in 20mL of dichloromethane, add N,N-diisopropylethylamine DIEA (0.516g, 4mmol), stir at 25°C for 20min, and slowly add tertiary bromoacetic acid to the reaction syst...
Embodiment 2
[0101] Preparation and Physicochemical Properties of Chlorambucil Betaine Conjugated Liposome Microspheres
[0102] Dissolve 0.1 g of the chlorambucil betaine conjugate of Example 1 in 20 mL of chloroform, evaporate the solvent at 60° C., add 10 mL of PBS (pH 7.4) solution, form a film at 60° C., and shake for 10 minutes to obtain phenbutylene Aqueous solution of mustardine betaine conjugated liposome microspheres. Particle Size Distribution and Zeta Potential of Chlorambucil Betaine Conjugated Lipid Granules See Figure 6 , see the morphology Figure 7 Transmission electron micrograph of chlorambucil betaine-conjugated liposomes.
Embodiment 3
[0104] Synthesis of camptothecin betaine conjugates (synthetic route see Figure 8 )
[0105] Camptothecin 0.5g, adipic anhydride 2.5g, DMAP 0.1g, triethylamine (Et 3 N) 1.5 g, dissolved in DMSO, heated to reflux for 12 hours; washed with 0.1M dilute hydrochloric acid for 3 times, filtered the dilute hydrochloric acid layer, and took the filter cake. Add 1.0 g of CDI, dissolve in 20 mL of dichloromethane, stir at 25°C for 2 h, and filter. Add 3-dimethylamino-1,2-propanediol (0.12g) and DBU (0.25g) to the filtrate, and heat to reflux for 24h. The reaction solution was diluted to 50mL with dichloromethane, washed 3 times with 1M hydrochloric acid, Na 2 SO 4 After drying, the solvent was evaporated to dryness to obtain intermediate product 1. The reaction process was detected by TLC (the developer was methanol:dichloromethane / 1:1, and the ultraviolet detection was λ254nm); the structure of the product was determined by mass spectrometry. MS:[M+H] + m / z, 1037.4.
[0106] 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com