Gefarnate key intermediate refining or reaction solution direct post-processing method
An intermediate, gefar ester technology, which is applied in the field of medicine, can solve the problems of cumbersome operation, low yield, and low purity of gefar ester, and achieve the effects of increasing yield, reducing losses, and simplifying operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
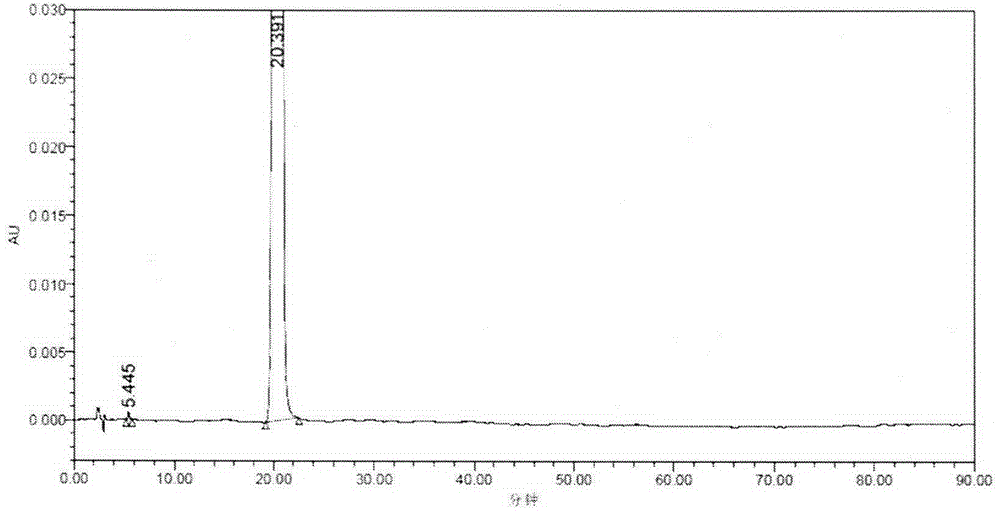
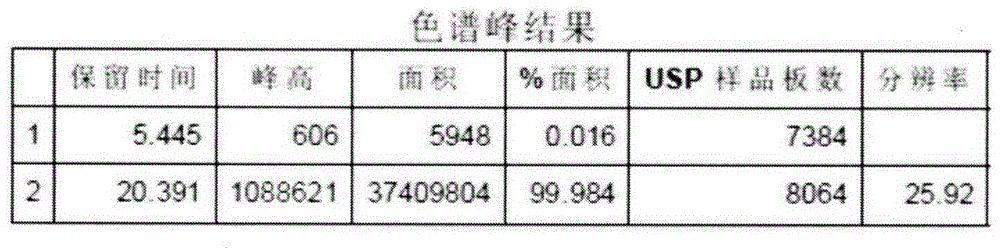
[0032] Post-treatment of the reaction solution: refer to the patent CN101805260A: add 292.5g (1.0mol) of intermediate (IV), 96.0g (1.7mol) of potassium hydroxide, and 1900ml of 95% ethanol to a 3000mL three-necked glass reaction bottle, heat up to reflux reaction, TLC Track the completion of the reaction (developer: ethyl acetate:petroleum ether=1:9; iodine color development), after the reaction is complete, concentrate under reduced pressure until no ethanol drips out. At room temperature, add 500ml of purified water and stir to dissolve evenly, add acetic acid Wash 250ml of ethyl ester for 3 times (remove fat-soluble impurities), remove the ethyl acetate layer, add 2.0g of medicinal charcoal to the water layer, stir at room temperature for 20 minutes for decolorization, filter for decarburization, remove water-insoluble impurities, adjust the pH of the water layer with dilute hydrochloric acid The value is 2, add 250ml of ethyl acetate to extract 3 times, combine the ethyl ac...
Embodiment 2
[0034]Aftertreatment of the reaction solution: refer to the patent CN1O1805260A: add 292.5g (1.0mol) of intermediate (IV), 96.0g (1.7mol) of potassium hydroxide, and 1900ml of 95% ethanol in a 3000mL three-necked glass reaction bottle, heat up and reflux, TLC Track the completion of the reaction (developer: ethyl acetate:petroleum ether=1:9; iodine color development), after the reaction is complete, concentrate under reduced pressure until no ethanol drips out. At room temperature, add 800ml of purified water and stir to dissolve evenly. Wash 3 times with 400ml of propyl ether (to remove fat-soluble impurities), remove the isopropyl ether layer, add 2.0g of medicinal charcoal to the water layer, stir at room temperature for 20 minutes for decolorization, filter for decarburization, remove water-insoluble impurities, and adjust the pH of the water layer with dilute hydrochloric acid value is 1, add 400ml of isopropyl ether to extract 3 times, combine the isopropyl ether layer, w...
Embodiment 3
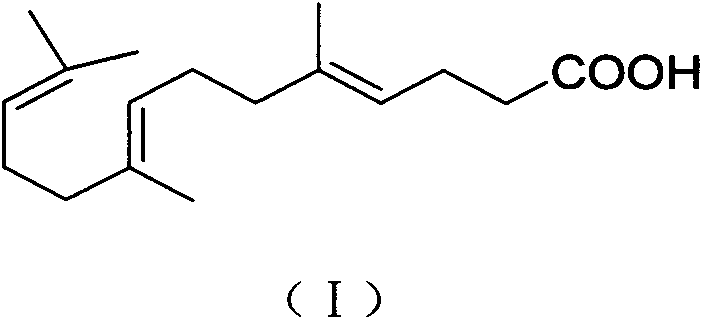
[0036] Refining: Add formula (I) compound E-farnesic acid crude product 200g in 3000mL three-necked glass reaction flask, add 15% potassium hydroxide 400ml under room temperature, stir and dissolve evenly, add ethyl acetate 250ml and wash 3 times (degreasing soluble impurities), remove the ethyl acetate layer, add 2.0 g of medicinal charcoal to the water layer, stir at room temperature for 20 minutes for decolorization, decarburize by filtration, remove water-insoluble impurities, adjust the pH value of the water layer to 3 with dilute hydrochloric acid, add 200 ml of ethyl acetate Extract 3 times, combine the ethyl acetate layers, wash 3 times with 200ml of water (to remove water-soluble impurities), add anhydrous sodium sulfate to the organic layer, dry, filter, and concentrate the filtrate under reduced pressure until no ethyl acetate is present, to obtain the compound of formula (I) E-farnesic acid was 227.6g, and the molar yield was 86.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com