Organic-ligand-based multifunctional zinc complexes and application thereof
A technology of organic ligands and zinc complexes, applied in the field of photocatalysis and fluorescent material synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1 Synthesis {[Zn(L 1 )(mip)]·H 2 O} n ,among them, L 1 for N , N '-Bis(3-pyridine)pyridine-2,6-dimethylformamide, the structural formula is: , Mip is 5-methyl isophthalate
[0074] Add 0.2mmol Zn(NO 3 ) 2 ·6H 2 O, 0.10mmol N , N '-Bis(3-pyridine)pyridine-2,6-dimethylamide, 0.2mmol 5-methylisophthalic acid and 5mL H 2 O was added to a 25mL beaker and stirred at room temperature for 10 minutes to obtain a suspension mixture. After adjusting the pH of the suspension mixture to 6.3 with 0.5mol / L NaOH solution, it was transferred to a 25mL autoclave at a temperature of 2.5℃ / h. The heating rate is increased to 100℃, and the temperature is kept for 36h under hydrothermal conditions, and the temperature is reduced to room temperature at a cooling rate of 10℃ / h to obtain colorless lumpy crystals, which are washed twice with deionized water and ethanol alternately, and allowed to dry naturally at room temperature Dry, get {[Zn(L 1 )(mip)]·H 2 O} n , The yield is 55%, and its...
Embodiment 2
[0075] Example 2 Synthesis {[Zn(L 1 )(mip)]·H 2 O} n ,among them, L 1 for N , N '-Bis(3-pyridine)pyridine-2,6-dimethylamide, mip is 5-methyl isophthalate
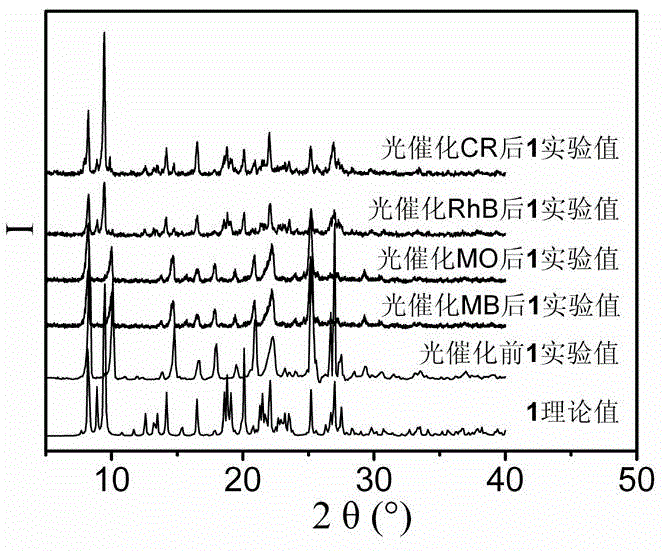
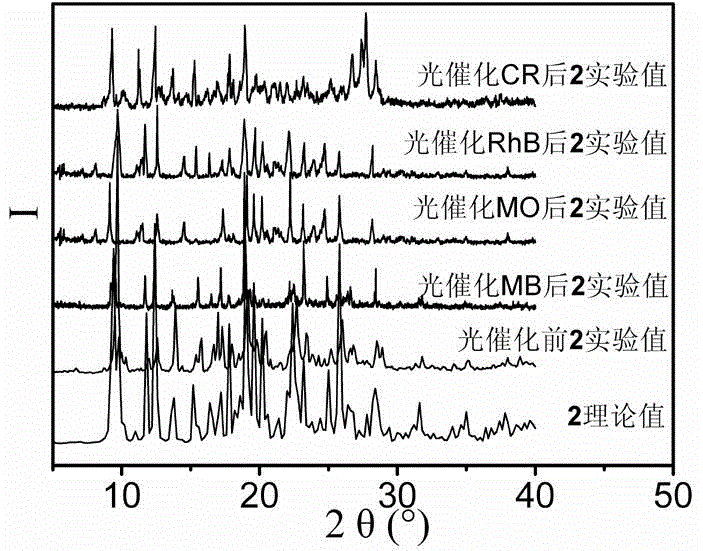
[0076] Add 0.1mmol Zn(NO 3 ) 2 ·6H 2 O, 0.10mmol N , N '-Bis(3-pyridine)pyridine-2,6-dimethylamide, 0.10mmol 5-methylisophthalic acid and 10mL H 2 O was added to a 25mL beaker in turn, stirred at room temperature for 40 minutes to obtain a suspension mixture. After adjusting the pH of the suspension mixture to 6.7 with 0.5mol / L NaOH solution, it was transferred to a 25mL autoclave at a temperature of 5℃ / h. The heating rate is increased to 120℃, and the temperature is kept for 96h under hydrothermal conditions. The temperature is reduced to room temperature at a cooling rate of 2.5℃ / h to obtain colorless block crystals, which are washed 4 times with deionized water and ethanol alternately, and allowed to dry naturally at room temperature Dry, get {[Zn(L 1 )(mip)]·H 2 O} n , The yield is 76%, and its XRD pattern is as figure 1 A...
Embodiment 3
[0077] Example 3 Synthesis {[Zn(L 1 )(mip)]·H 2 O} n ,among them, L 1 for N , N '-Bis(3-pyridine)pyridine-2,6-dimethylamide, mip is 5-methyl isophthalate
[0078] Add 0.3mmol Zn(NO 3 ) 2 ·6H 2 O, 0.10mmol N , N '-Bis(3-pyridine)pyridine-2,6-dimethylamide, 0.15mmol 5-methylisophthalic acid and 7.5mL H 2 O was added to a 25mL beaker and stirred for 50min at room temperature to obtain a suspension mixture. After adjusting the pH of the suspension mixture to 7.2 with 1mol / L NaOH solution, it was transferred to a 25mL autoclave and heated at 5℃ / h. The rate is increased to 140℃, kept for 48h under hydrothermal conditions, and the temperature is lowered to room temperature at a cooling rate of 5℃ / h to obtain colorless lumpy crystals, which are washed 5 times with deionized water and ethanol alternately, and dried naturally at room temperature , Get {[Zn(L 1 )(mip)]·H 2 O} n , The yield is 60%, and its XRD diffraction pattern is as figure 1 As shown, the coordination environment diagram ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com