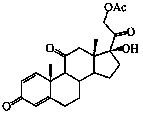
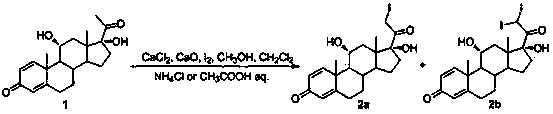Preparation method of prednisone acetate
A technology of prednisone acetate and its products, which is applied in the field of preparation of steroid drugs, can solve the problems of affecting the purity and quality of the product, not conforming to atom economy, and difficult to complete conversion, etc., and achieves low cost, high yield, and high selection sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of prednisone acetate of the present invention may further comprise the steps:
[0030] The first step, iodination reaction:
[0031] Process 1.1, preparation of iodine solution: anhydrous CaCl 2 Dissolve in methanol, avoid light, add iodine, and set aside;
[0032] Among them, iodine and CaCl 2 The mass ratio is 1:0.1~1:0.4, anhydrous CaCl 2 The molar concentration of the methanol solution is between 0.24 and 3.6 mol / L;
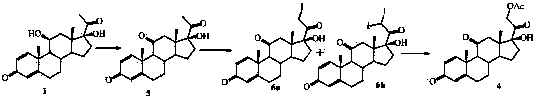
[0033] Step 1.2, adding anhydrous CaCl to the reaction bottle 2 and methanol, stir to dissolve, add solvent, dihydroxyprogesterone dehydrogenate 1, cool down to 0-10°C, add CaO, keep away from light, drop in iodine solution at -5-10°C, add dropwise for 2-4 hours After the dropwise addition, the reaction was continued at -5-10°C for 4-6 hours, and the reaction was stopped when there was no raw material point detected by TLC; the reaction was quenched by adding 5-30% ammonium chloride or 5-30% acetic acid aqueous solution, an...
Embodiment 1
[0058] The first step, iodination reaction:
[0059] Process 1.1. Preparation of iodine solution: add CaCl to a 500mL one-mouth bottle 2 22g and 220mL of methanol, stirred to dissolve, protected from light, added 95g of iodine, stirred for later use;
[0060] Step 1.2, add anhydrous CaCl to the 2000mL four-necked reaction flask 2 10g, 100mL of methanol, stir to dissolve, add 100g of dihydroxyprogesterone dehydrogenate, 500mL of chloroform, cool down to 0-10°C, add 65g of CaO, keep away from light, cool down to -5-0°C, add iodine solution dropwise, After 2 to 3 hours of dropping, continue to react at -5 to 0°C for 4 to 5 hours after the dropping, stop the reaction when there is no raw material point detected by TLC; drop 5 to 30% ammonium chloride aqueous solution to quench the reaction, and decompress Concentrate, pour into ice water and stir for 1 hour, suction filter to dryness, and vacuum-dry at 25°C to obtain the above iodine products 2a and 2b, with a molar yield of 9...
Embodiment 2
[0067] The first step, iodine reaction;
[0068] Process 1.1. Preparation of iodine solution: add CaCl to a 200mL one-mouth bottle 2 8g and 300mL of methanol, stir to dissolve, avoid light, add 80g of iodine, stir for later use;
[0069] Step 1.2, add anhydrous CaCl to the 2000mL four-necked reaction flask 2 5.5g, 55mL of methanol, stir to dissolve, add 100g of dihydroxyprogesterone dehydrogenate, 165mL of dichloromethane, cool down to 0-10°C, add 50g of CaO, keep away from light, drop the iodine solution at 0-10°C, After 2-3 hours of dropping, continue the reaction at 0-10°C for 5-6 hours after the dropping, stop the reaction when there is no raw material point detected by TLC, drop 5-30% acetic acid aqueous solution to quench the reaction, concentrate under reduced pressure, pour Stir in ice water for 1 hour, suction filter to dryness, and vacuum-dry at 25°C to obtain the above iodine products 2a and 2b, with a molar yield of 95% and an HPLC content of 96%.
[0070] The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com