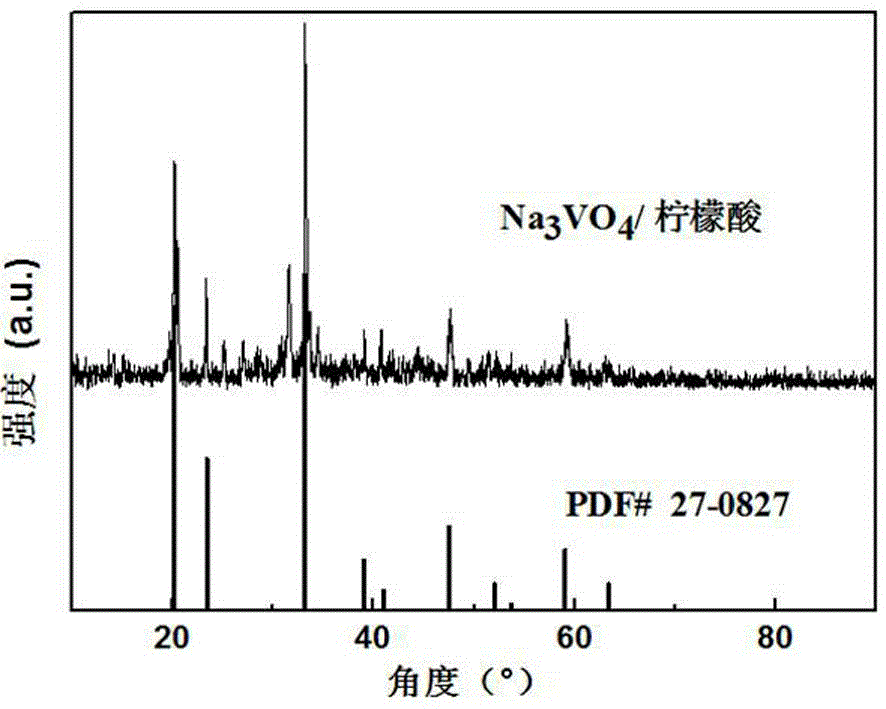
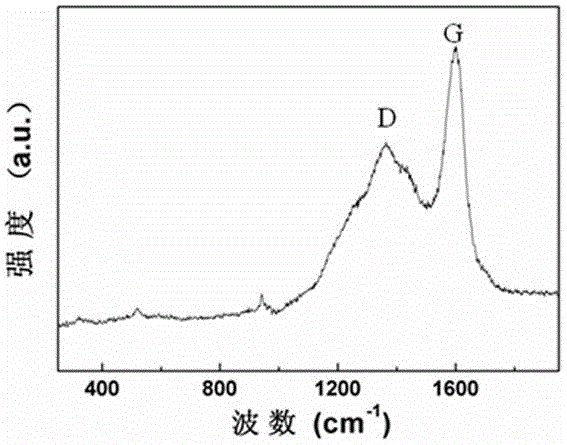
Composite lithium ion battery anode material and preparation method thereof
A negative electrode material and ion battery technology, applied in the field of electrochemical power supply, can solve problems such as unsatisfactory conductivity and poor material rate performance, and achieve the effects of low cost, good cycle performance, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The material synthesis steps are as follows:
[0027] 1) Weigh 3mmol and 1mmol of sodium carbonate and vanadium pentoxide according to the molar ratio of 3:1 and dissolve them in beakers A and B filled with 10ml of distilled water, and stir on a magnetic stirrer for 20min to fully dissolve;
[0028] 2) Weigh 5mmol of hexamethylenetetramine and dissolve it in beaker C filled with 10ml of distilled water, transfer the solutions in beakers B and C in step 1) to beaker A, stir on a magnetic stirrer for 20min to obtain a uniform color solution;
[0029] 3) Transfer the uniformly colored solution obtained in step 2) to the lining of a 50ml hydrothermal kettle to 80% volume, react in a blast oven at 120°C for 24 hours, and cool naturally to room temperature;
[0030] 4) adding citric acid with a theoretical carbon content of 10% to the product obtained in step 3);
[0031] 5) The intermediate product obtained in step 4) was dried in an oven at 80°C for 12 hours, and then cal...
Embodiment 2
[0034] The material synthesis steps are as follows:
[0035] 1) Weigh 3 mmol and 1 mmol of sodium hydroxide and ammonium metavanadate according to the molar ratio of 3:1, respectively, and dissolve them in beakers A and B filled with 10 ml of distilled water, and stir on a magnetic stirrer for 20 minutes to fully dissolve;
[0036] 2) Weigh 5mmol of hexamethylenetetramine and dissolve it in beaker C filled with 10ml of distilled water, transfer the solutions in beakers A and B in step 1) to beaker C, stir on a magnetic stirrer for 20min to obtain a uniform color solution;
[0037] 3) Transfer the uniformly colored solution obtained in step 2) to the lining of a 50ml hydrothermal kettle to 80% volume, react in a blast oven at 120°C for 24 hours, and cool naturally to room temperature;
[0038] 4) adding glucose with a theoretical carbon content of 10% to the product obtained in step 3);
[0039] 5) The intermediate product obtained in step 4) was dried in an oven at 80°C for ...
Embodiment 3
[0042] The material synthesis steps are as follows:
[0043] 1) Weigh 3mmol and 1mmol of sodium carbonate and vanadium pentoxide according to the molar ratio of 3:1 and dissolve them in beakers A and B filled with 10ml of distilled water, and stir on a magnetic stirrer for 20min to fully dissolve;
[0044] 2) Weigh 5mmol of hexamethylenetetramine and dissolve it in beaker C with 10ml of distilled water, transfer the solution in beaker C in step 1) to B and step 1) to beaker A, and stir on a magnetic stirrer for 20min Obtain a solution of uniform color;
[0045] 3) Transfer the uniformly colored solution obtained in step 2) to the lining of a 50ml hydrothermal kettle to 80% volume, react in a blast oven at 120°C for 24 hours, and cool naturally to room temperature;
[0046] 4) adding sucrose with a theoretical carbon content of 10% to the product obtained in step 3);
[0047] 5) The intermediate product obtained in step 4) was dried in an oven at 80°C for 12 hours, and then c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com