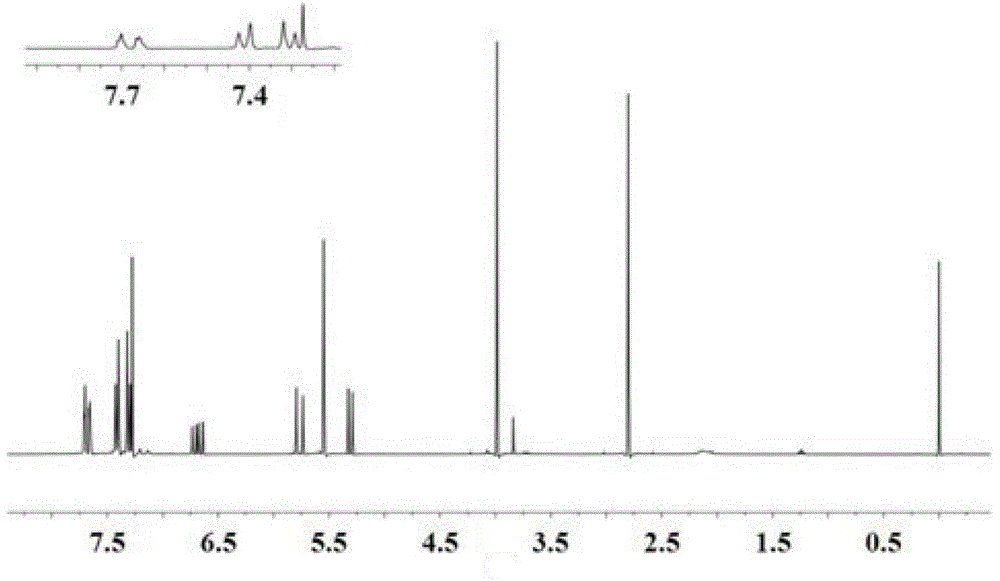
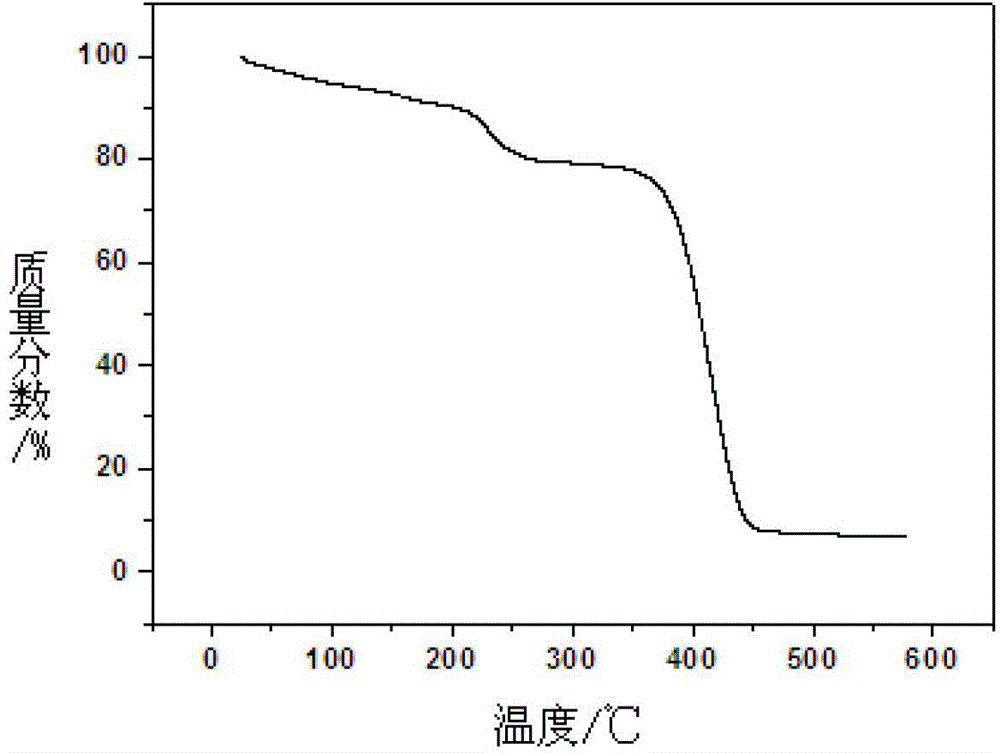
Imidazolyl ionic liquid and application thereof in alkaline anion exchange membrane
A basic anion and ionic liquid technology, which is applied in the preparation of the above-mentioned membranes, and the field of imidazole-based cross-linked basic anion-exchange membranes. , easy to form carbene and other problems, to achieve good ion-conducting ability, uniform surface, and enhanced stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Add 5mL ethanol, 5mL p-chloromethylstyrene (density 1.08g / cm 3 , molecular weight 152.5g / mol) and 3.8g 1-methyl-2-ethylimidazole (molecular weight 110g / mol), magnetically stirred, and heated in an oil bath at 70°C for 6h, the above solution turned orange. After returning to room temperature, the obtained orange solution was added to 250 mL of ethyl acetate to obtain a light yellow solid imidazolium salt, which was filtered and dried to constant weight before use. Take 1g of imidazolium salt, dissolve it with 5mL dimethyl sulfoxide, add styrene in an amount 3 times that of imidazolium salt, and then add 1% of the sum of the amount of imidazolium salt and styrene Divinylbenzene, the amount of the last added substance is the free radical initiator azobisisobutyronitrile of 1% of the sum of the amount of imidazolium salt and styrene substance; after magnetic stirring is mixed uniformly, freeze-thaw cycle is carried out, and the freezing process In order to freeze the react...
Embodiment 2
[0062] Add 10mL of acetonitrile and 3mL of p-chloromethylstyrene (density 1.08g / cm 3 , molecular weight 152.5g / mol) and 1.9g 1-ethyl-2-hexylimidazole (molecular weight 180g / mol), magnetically stirred and heated in an oil bath at 60°C for 12h, the above solution turned orange. After returning to room temperature, the obtained orange solution was added to 200 mL ethyl acetate to obtain a light yellow solid imidazolium salt, which was filtered and dried to constant weight for later use. Take 1g of imidazolium salt, dissolve it with 5mL dimethyl sulfoxide, add styrene in an amount 4 times that of imidazolium salt, and then add 3% of the sum of imidazolium salt and styrene Divinylbenzene, the amount of the last added substance is 3% of the free radical initiator azobisisobutyronitrile of the sum of the amount of imidazolium salt and styrene substance, and magnetic stirring is mixed to carry out freezing-thawing cycle, freezing process In order to freeze the reaction tube containin...
Embodiment 3
[0065] Into a 100mL reaction tube, add 30mL dimethylformamide and 3mL p-chloromethylstyrene (density 1.08g / cm 3 , molecular weight 152.5g / mol) and 8.8g 1-hexyl-2-butylimidazole (molecular weight 208g / mol), magnetically stirred, and heated in an oil bath at 70°C for 6h, the above mixture turned orange. After returning to room temperature, the obtained orange solution was added to 350 mL of ethyl acetate to obtain a light yellow solid imidazolium salt, which was filtered and dried to constant weight before use. Get 1g of imidazolium salt, dissolve it with 5mL of absolute ethanol and add styrene with a ratio of 1:2 to the amount of substance of imidazolium salt, then add the amount of substance that is 4% of the sum of the amount of substance of imidazolium salt and styrene % of divinylbenzene, the amount of the last added substance is the 1% free radical initiator dibenzoyl peroxide of the sum of the amount of imidazolium salt and styrene substance, and magnetic stirring is mixe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum power density | aaaaa | aaaaa |
| Current density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com