A dopo-containing bismaleimide with asymmetric molecular structure, its preparation method and its application in the preparation of composite resin
A bismaleimide and molecular structure technology, applied in the field of microelectronic packaging materials, can solve the problems of reducing resin performance, reducing heat resistance, and being difficult to meet flame retardant requirements, and achieving the effect of solving poor solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Preparation of asymmetric dibasic primary amines containing DOPO
[0048] The asymmetric dibasic primary amine containing DOPO represented by the general formula (I) is prepared by referring to the method disclosed in the existing literature (Journal of PolymerScience: Part A: Polymer Chemistry, Vol.49, 1331-1340 (2011)) , the synthetic route is shown in the following formula:
[0049]
[0050] Among them, R 1 is a hydrogen atom, C 1 -C 6 Alkyl, C 1 -C 6 Alkoxy, -CF 3 or a halogen atom;
[0051] R 2 is a hydrogen atom, methyl, 2,6-dimethyl, methoxy or allyl;
[0052] R3 is a hydrogen atom, methyl, 2,6-dimethyl, ethyl or methoxy;
[0053] R 4 is a hydrogen atom, C 1 -C 6 Alkyl, -CF 3 or benzene ring;
[0054] X is O or S;
[0055] x 1 is OH or SH;
[0056] x 2 is F or Cl;
[0057] with R 1 , R 2 and R 3 Take hydrogen atom, R respectively 4 Take -CH 3 , X takes O, X 1 Take OH, X 2 Taking F as an example, the detailed synthetic route ...
Embodiment 2
[0062] Example 2 Preparation of Bismaleimide Monomer Containing DOPO and Asymmetric Molecular Structure by Method 1
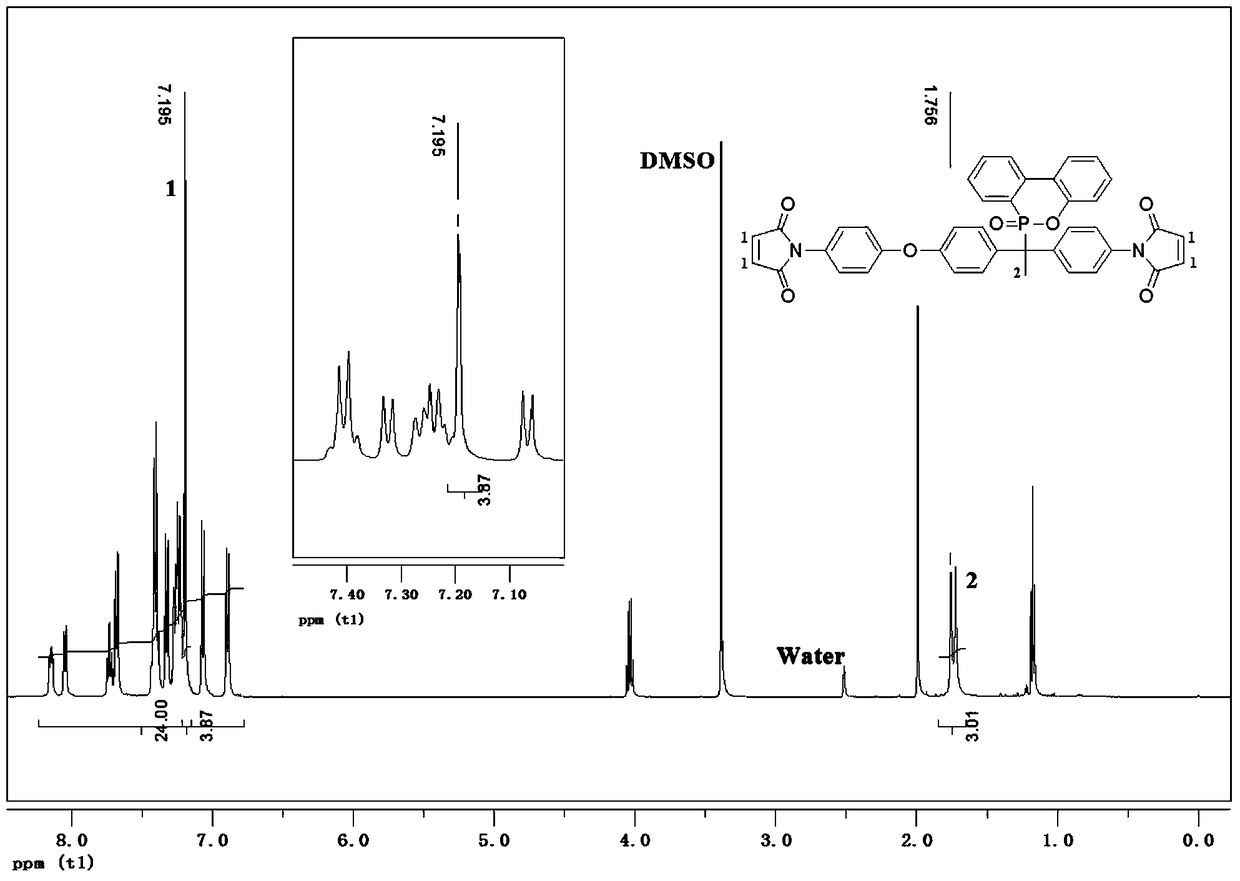
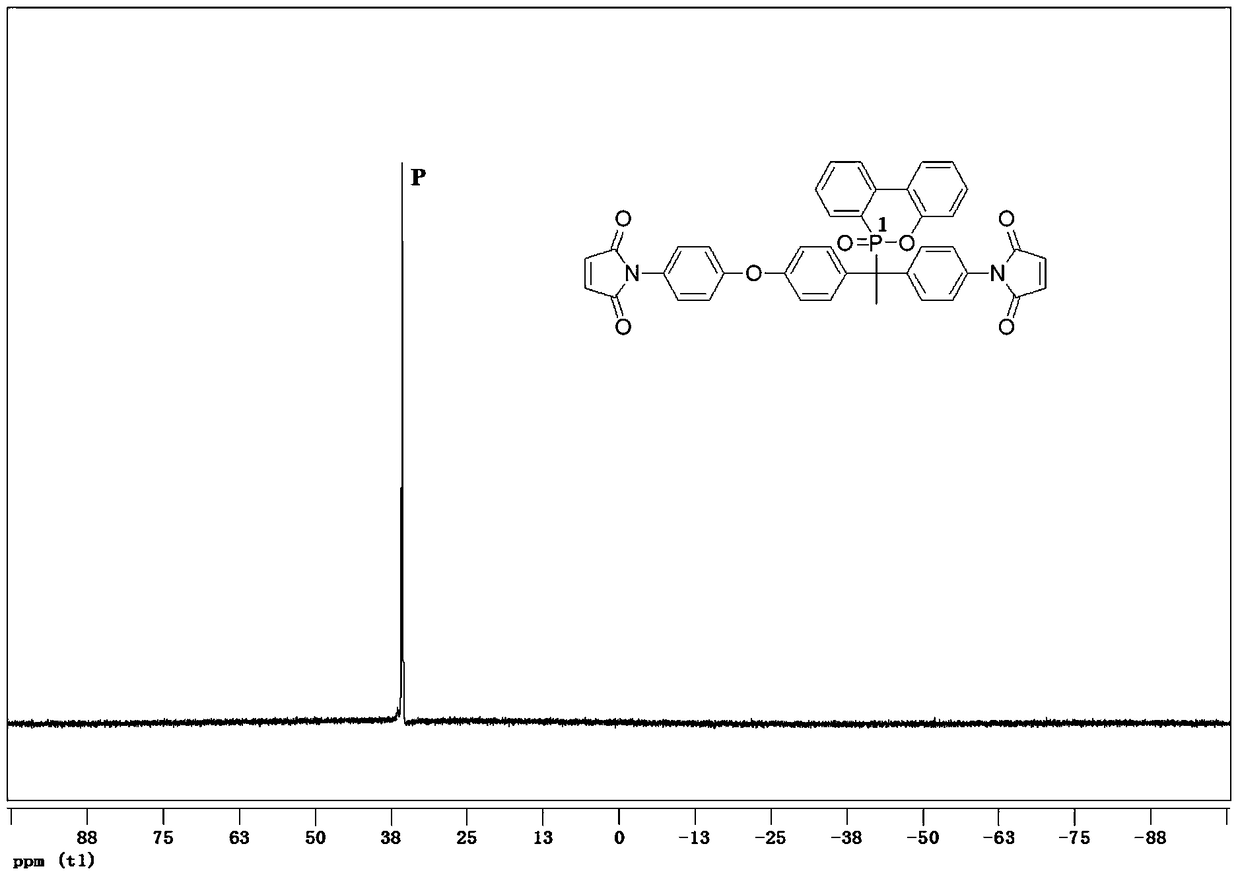
[0063] with R 1 , R 2 , R 3 and R 5 Take hydrogen atom, R respectively 4 Take -CH 3 , X takes O, and the mixed solvent containing toluene takes toluene / DMF (volume ratio is 2 / 1) mixed solution as example, by the first kind of preparation method provided by the present invention, with the formula (I- 1) The asymmetric dibasic primary amine containing DOPO shown in 1) prepares the compound shown in the following formula (II-1):
[0064] 1-(4'-maleimidophenyl)-1-(4'-(4"-maleimidophenoxy)phenyl-1-(10'-(9',10 '-Dihydro-9'-oxa-10'-phosphaphenanthrene-10'-oxide) ethane,
[0065] 1-(4'-maleimido)phenyl-1-[4'-(4”-maleimido)phenoxyl]phenyl-1-[(9,10-dih ydro-9-oxa-10-phosphaphenanthrene)-10-yl] ethane:
[0066]
[0067] Weigh 5.18g (0.01mol) of the formula (I-1) prepared in Example 1 containing DOPO dibasic primary amine, and add 1.96g (0.02mol) of maleic anhy...
Embodiment 3
[0070] Embodiment 3 Further preparation of cyanate ester composite resin with the monomers prepared in Example 2
[0071] The bismaleimide monomer containing DOPO shown in the formula (II-1) prepared in Example 2 is used to prepare BT composite resin, the cyanate is bisphenol A cyanate, and no allylphenols are added. Compounds, epoxy resins, catalysts:
[0072] Weigh 80 parts by mass of the bismaleimide shown in formula (II-1) prepared in Example 1 and 20 parts by mass of bisphenol A cyanate, melt and blend for 10 minutes at 140 ° C and immediately pour into aluminum and degassed at 180°C. After no obvious air bubbles, cured at 230°C for 2 hours and 300°C for 8 hours to obtain cured BT resin.
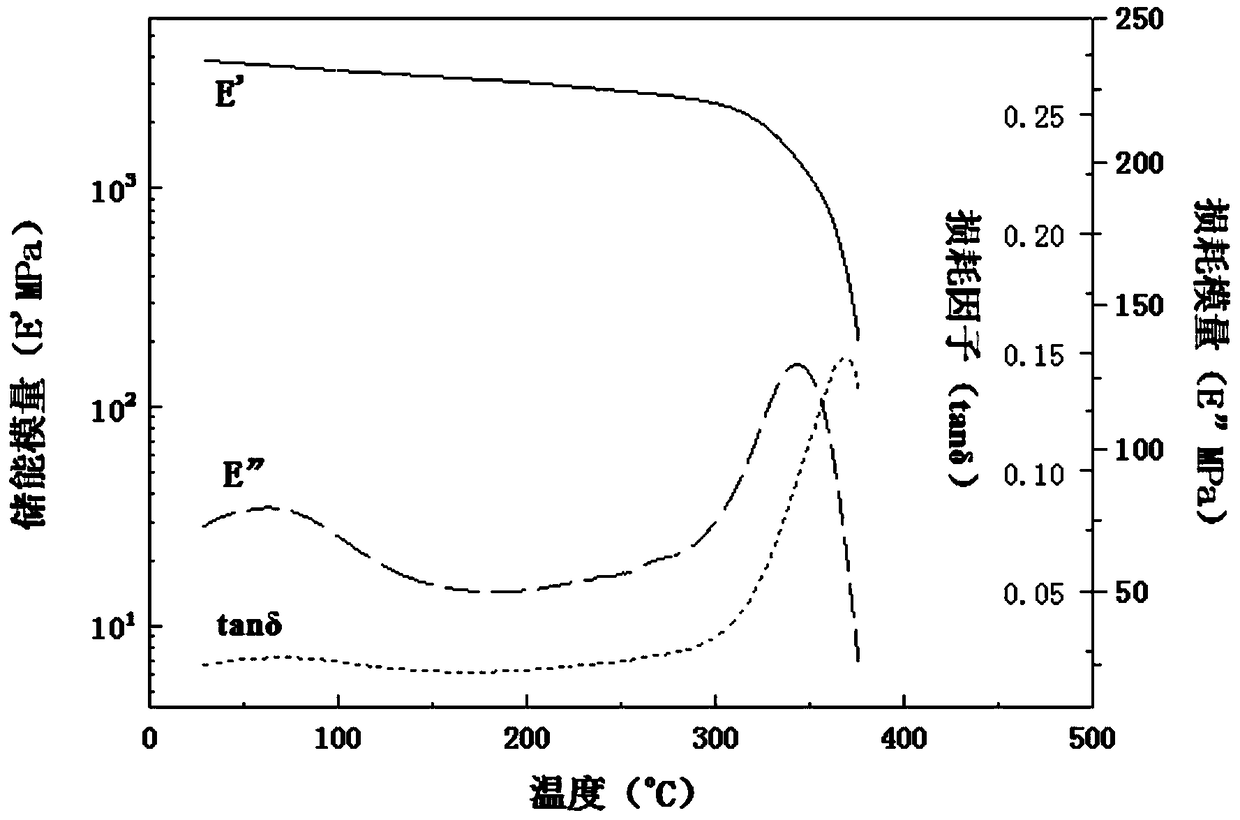
[0073] The BT composite resin prepared in this example has good solubility and can be dissolved in low boiling point and low toxicity solvents such as methanol, acetone, methylene chloride and tetrahydrofuran, and has high modulus and high glass transition temperature after curing , the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| storage modulus | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com