Preparation of carbodithioic acid-modified heavy metal adsorbing material and application of material for removing Cu<2+> in wastewater
A technology of dithiocarboxylic acid and adsorption material, which is applied in the fields of adsorption water/sewage treatment, water pollutants, water/sewage treatment, etc. The effect of alkali resistance and simple preparation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
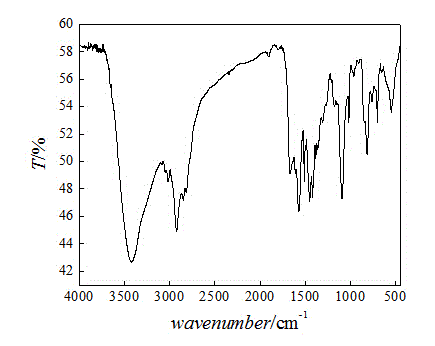
[0030] (1) Extract a certain amount of chlorine balls in a Soxhlet extractor with absolute ethanol for 8 hours. After extraction, the chlorine balls are washed repeatedly with ultrapure water and absolute ethanol until the filtrate is a colorless solid and odorless. Dry in an oven at 60°C and store in a desiccator;
[0031] (2) Take 5g of the extracted chlorine spheres in a 250mL three-neck round bottom flask, add 30mL DMF to swell for 12 hours. Under the condition of magnetic stirring, 5 mL of diethylenetriamine was slowly added at 45° C., and the reaction was carried out under constant temperature reflux for 12 hours. After the reaction, the product was repeatedly washed with ultrapure water and absolute ethanol until the filtrate was a colorless solid and odorless. The grafting rate of the amino-based microsphere of gained is 31.1%;
[0032] (3) Swell the amine-based microspheres in DMF for 12 hours, slowly add carbon disulfide at 45°C, and react at constant temperature f...
Embodiment 2
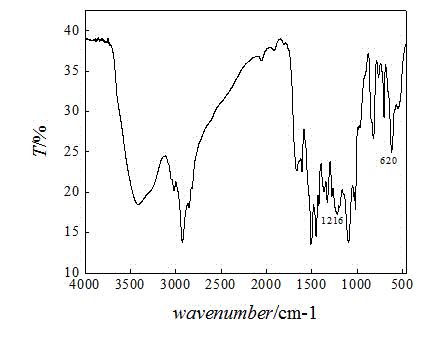
[0035] (1) Extract a certain amount of chlorine balls in a Soxhlet extractor with absolute ethanol for 8 hours. After extraction, the chlorine balls are washed repeatedly with ultrapure water and absolute ethanol until the filtrate is a colorless solid and odorless. Dry in an oven at 60°C and store in a desiccator;
[0036] (2) Take 5g of the extracted chlorine spheres in a 250mL three-neck round bottom flask, add 30mL DMF to swell for 12 hours. Under the condition of magnetic stirring, 15 mL of diethylenetriamine was slowly added at 45° C., and the reaction was carried out under constant temperature reflux for 12 hours. After the reaction, the product was repeatedly washed with ultrapure water and absolute ethanol until the filtrate was a colorless solid and odorless. The grafting rate of the amino-based microsphere of gained is 36.9%;
[0037] (3) Swell the amine-based microspheres in DMF for 12 hours, slowly add carbon disulfide at 45°C, and react at constant temperature ...
Embodiment 3
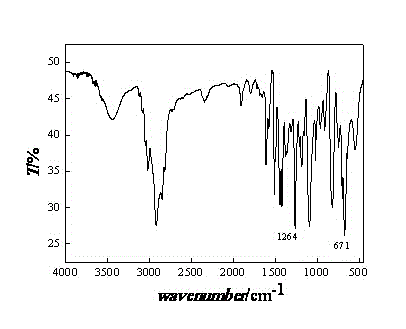
[0040] (1) Extract a certain amount of chlorine balls in a Soxhlet extractor with absolute ethanol for 8 hours. After extraction, the chlorine balls are washed repeatedly with ultrapure water and absolute ethanol until the filtrate is a colorless solid and odorless. Dry in an oven at 60°C and store in a desiccator;
[0041] (2) Take 5g of the extracted chlorine spheres in a 250mL three-neck round bottom flask, add 30mL DMF to swell for 12 hours. Under the condition of magnetic stirring, 20 mL of diethylenetriamine was slowly added at 45° C., and the reaction was carried out under constant temperature reflux for 12 hours. After the reaction, the product was repeatedly washed with ultrapure water and absolute ethanol until the filtrate was a colorless solid and odorless. The grafting rate of the amino-based microsphere of gained is 35.0%;
[0042](3) Swell the amine-based microspheres in DMF for 12 hours, slowly add carbon disulfide at 45°C, and react at constant temperature f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com