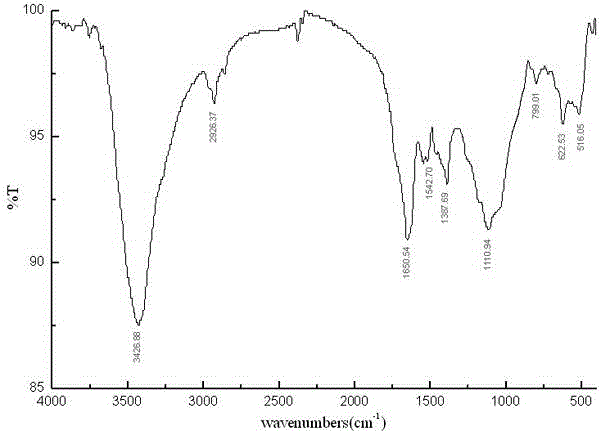
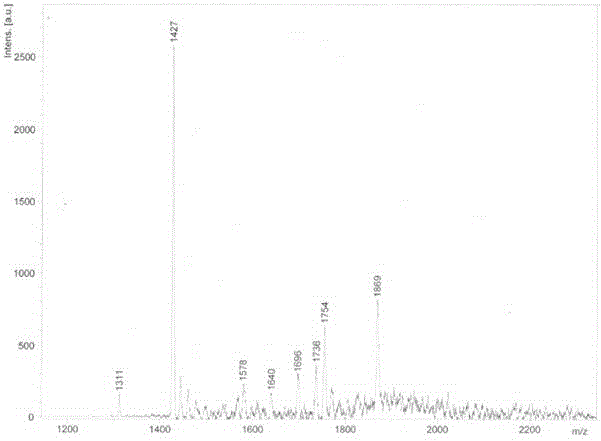
Method for synthetizing fullerene bis-addition polypeptide by combining liquid phase and solid phase
A double addition, fullerene technology, applied in the field of medicine, can solve the problems of unreported anti-tumor activity research, complex compound structure, etc., to achieve good anti-cancer activity, good free radical scavenging, and improved solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
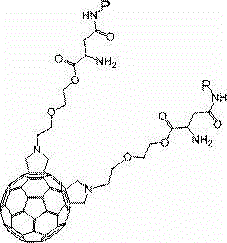
[0031] The synthetic route of the fullerene double addition RGD peptide of this embodiment is:
[0032]
[0033] Among them, 1 is N-substituted fullerene double-added pyrrolidine, 2 is fullerene double-added aspartic acid (including Fmoc and OtBu), 3 is fullerene double-added aspartic acid (containing Fmoc), 4 is fullerene double addition RGD peptide.
[0034] The preparation method of the fullerene double addition RGD peptide of the present embodiment is as follows:
[0035] (1) Take 0.24 mmol α-amino and β-carboxyl protected Fmoc-Asp-OtBu, dissolve 0.24 mmol HOBT in 4 mL DCM, add DMF drop by drop until the solution is clear, then add 0.24 mmol DCC and mix well, then protect the ice bath from light with nitrogen React for 30 minutes, filter out the white DCU, then add 0.012 mmol fullerene double-addition pyrrolidine and 3 mg DMAP, protect under nitrogen and avoid light, react for 72 hours, spin evaporate, and separate and purify by silica gel column chromatography, the el...
Embodiment 2
[0041] The preparation method of the fullerene double addition TISWPPR peptide of the present embodiment is as follows:
[0042] (1) Take 0.24mmol α-amino and β-carboxyl protected Fmoc-Glu-OtBu, dissolve 0.24mmol HOBT in 4mL DCM, add DMF drop by drop until the solution is clear, then add 0.24mmol DCC and mix well, then protect the ice bath from light with nitrogen After reacting for 30 minutes, filter off the white DCU, then add 0.024 mmol fullerene double-added pyrrolidine and 3 mg DMAP, protect against light under nitrogen, and react for 48 hours, then rotary evaporate, and separate and purify by silica gel column chromatography, the eluent is toluene / ethyl acetate =3:1, the main product obtained after separation is vacuum-dried to obtain product 2 after rotary evaporation;
[0043] (2) Product 2 was decarboxylated with 90% TFA / DCM, reacted at room temperature for 0.5h, and finally purified by cold ether precipitation to obtain fullerene double-added glutamic acid FBPG (cont...
Embodiment 3
[0046] The preparation method of the fullerene double addition PHLATLF peptide of the present embodiment is as follows:
[0047] (1) Take 0.24 mmol α-amino and β-carboxyl protected Fmoc-Asp-OtBu, dissolve 0.24 mmol HOBT in 4 mL DCM, add DMF drop by drop until the solution is clear, then add 0.24 mmol DCC and mix well, then protect the ice bath from light with nitrogen React for 30 minutes, filter off the white DCU, then add 0.006 mmol fullerene double-added pyrrolidine and 3 mg DMAP, protect against light under nitrogen, and react for 24 hours, then rotary evaporate, and separate and purify by silica gel column chromatography, the eluent is toluene / ethyl acetate =3:1, the main product obtained after separation is vacuum-dried to obtain product 2 after rotary evaporation;
[0048] (2) Product 2 was decarboxylated with 17% TFA / DCM, reacted at room temperature for 12 hours, and finally purified by cold ether precipitation to obtain fullerene double-added aspartic acid FBPD (inclu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com