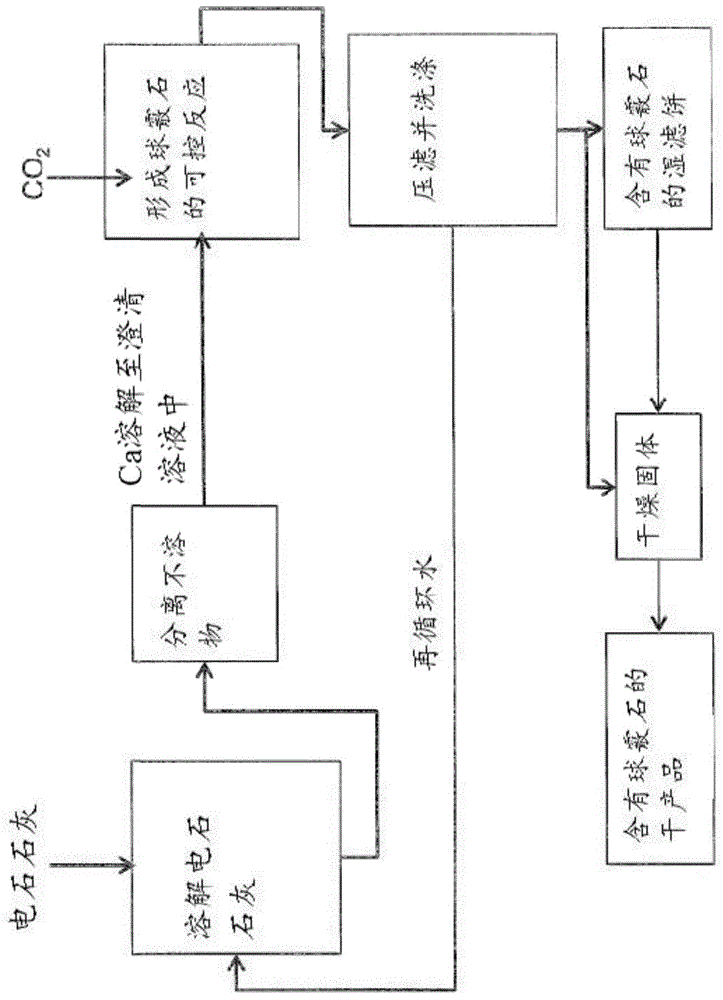
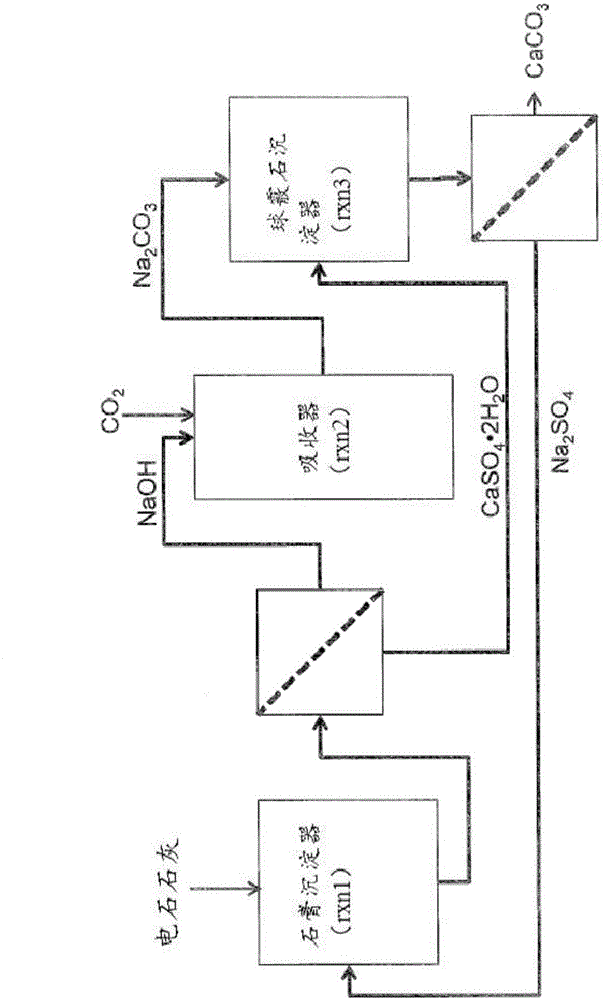
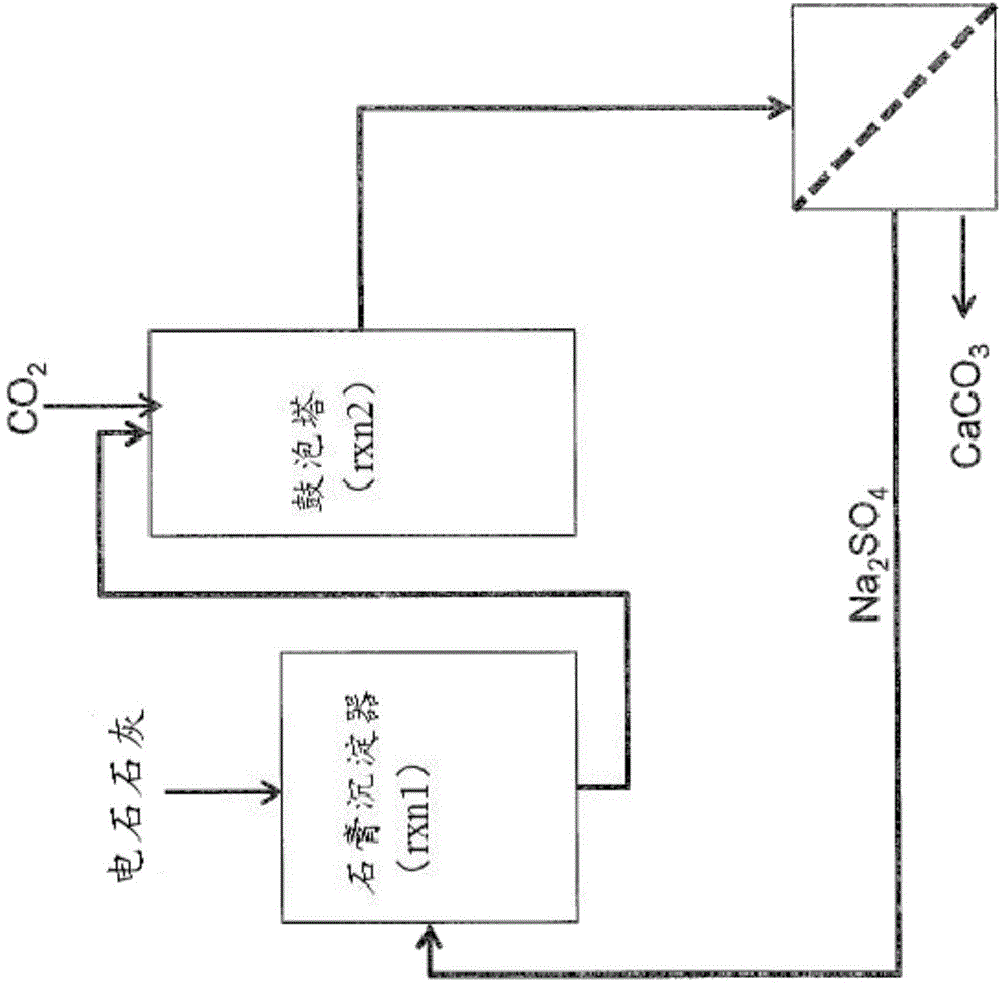
Methods and systems for utilizing carbide lime
A technology of lime and calcium carbide, applied in chemical instruments and methods, cement production, preparation of alkali metal carbonate shapes, etc., can solve problems such as environmental hazards and high economic costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0303] Purification of calcium carbide lime, formation and transformation of precipitated material
[0304] 1.88kg of NH 4 Cl was dissolved in 20.0L tap water. To this solution was added 1.18 kg carbide lime (~85% Ca(OH) 2 ), and mixed for 2 hours. The resulting mixture was vacuum filtered to remove insoluble impurities. Transfer the clarified filtrate to an airtight, collapsible bag. The bag was submerged in a water bath which preheated the solution to 35°C. The carbonation reactor is an acrylic cylinder equipped with baffles, gas diffusers, pH electrodes, thermocouples, turbine impellers, and liquid, gas, and slurry inlets and outlets. Mass flow controller controls N 2 -CO 2 Inlet gas mixing ratio. At start-up, 1 L of the solution in the bag was pumped into the reactor. Introducing CO through a gas diffuser 2 and N 2 While mixing the gas mixture, stir the mixture. A computer-automatically controlled loop controls a continuous input flow of fresh reactant solution...
Embodiment 2
[0307] Purification of calcium carbide lime and formation of precipitated material
[0308] 35.05g glycine (NH 2 CH 3 COOH) was dissolved in 1000 g deionized water. To this solution was added 17.30 g of calcium hydroxide (Ca(OH) 2 ). The mixture was stirred for 30 minutes. The mixture was vacuum filtered through Whatman No. 1 filter paper. At a temperature of 21.5°C, the pH of the resulting filtrate was 10.87. The filtrate was transferred to a 1 liter (4.5" inner diameter) batch reactor vessel with baffles. The filtrate was mixed with a 1.5" diameter rushton impeller at 2500 rpm while being fed with 1 slpm of carbon dioxide (CO 2 ) and 2slpm nitrogen (N 2 ) is bubbled through the reactor. When the pH reached 7.5 after 11 minutes and 25 seconds, the mixing and gas sparging was stopped. The resulting slurry was vacuum filtered through Whatman No. 1 filter paper. The solid filter cake was dried overnight at 100°C. 15.52 g of dry solid were recovered. The solid showed ...
Embodiment 3
[0310] Effect of Additives on Preparation of Aragonite Precipitated Materials with Low Density and High Porosity
[0311] In this experiment, an admixture was added to a precipitated material containing reactive vaterite to form a low-density and high-porosity aragonite microstructure suitable for lightweight and insulating applications such as drywall and ceiling tiles.
[0312] By capturing CO from flue gas 2 to produce calcium carbonate cement. In this process, in the absorber, the CO-containing 2 The raw flue gas is contacted with an alkaline aqueous solution to form a carbonated solution. Then, the carbonated solution was mixed with CaCl 2 Aqueous solution contact and add NaSO 4 acts as a stabilizer, leading to the metastable state of CaCO 3 Precipitation in the form of vaterite, followed by dehydration and drying of the precipitate yields the final carbonate precipitation material powder. The carbonate precipitation material showed 83% by weight vaterite and 17% by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com