Preparation method of porous carbon
A technology of porous carbon and organic matter, applied in the field of preparation of porous carbon, can solve the problems of difficult recovery of template agent and pore-forming agent, high cost of chemical corrosion resistance, complicated preparation process, etc., achieves good electrochemical performance, reduces energy consumption, The effect of simplifying the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation of porous carbon
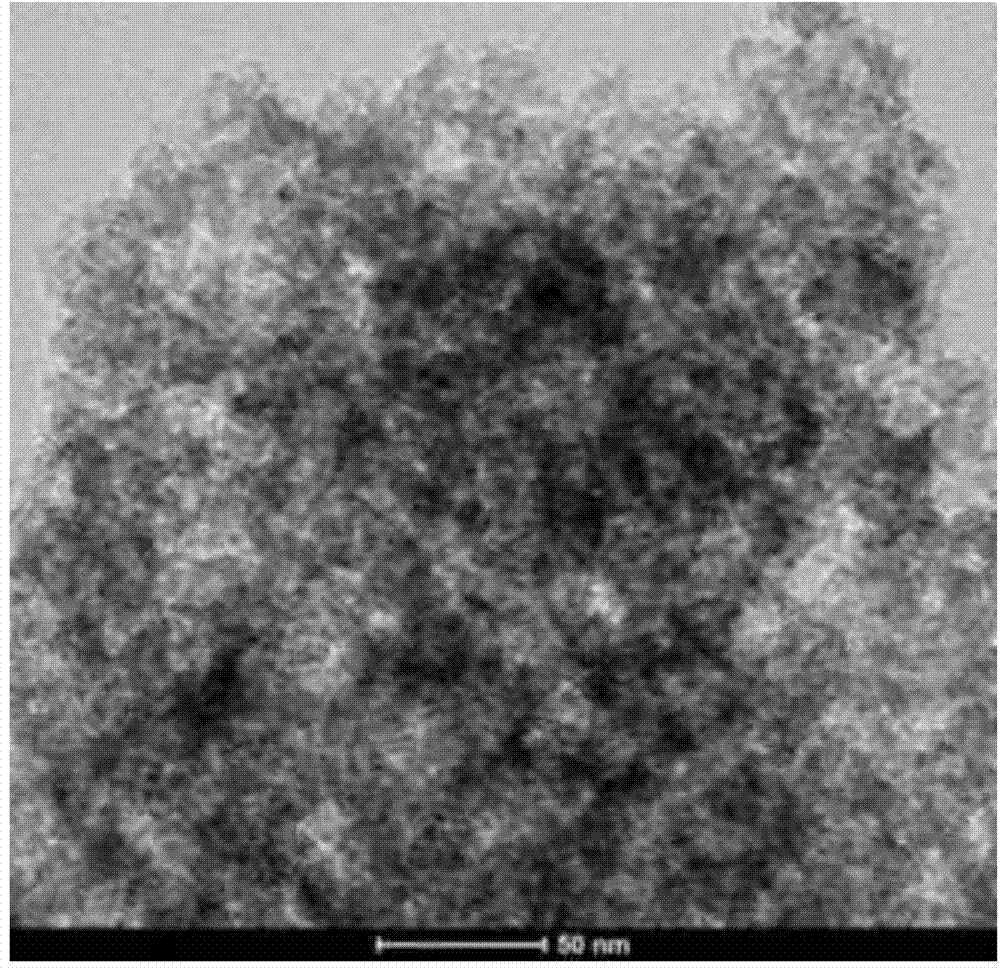
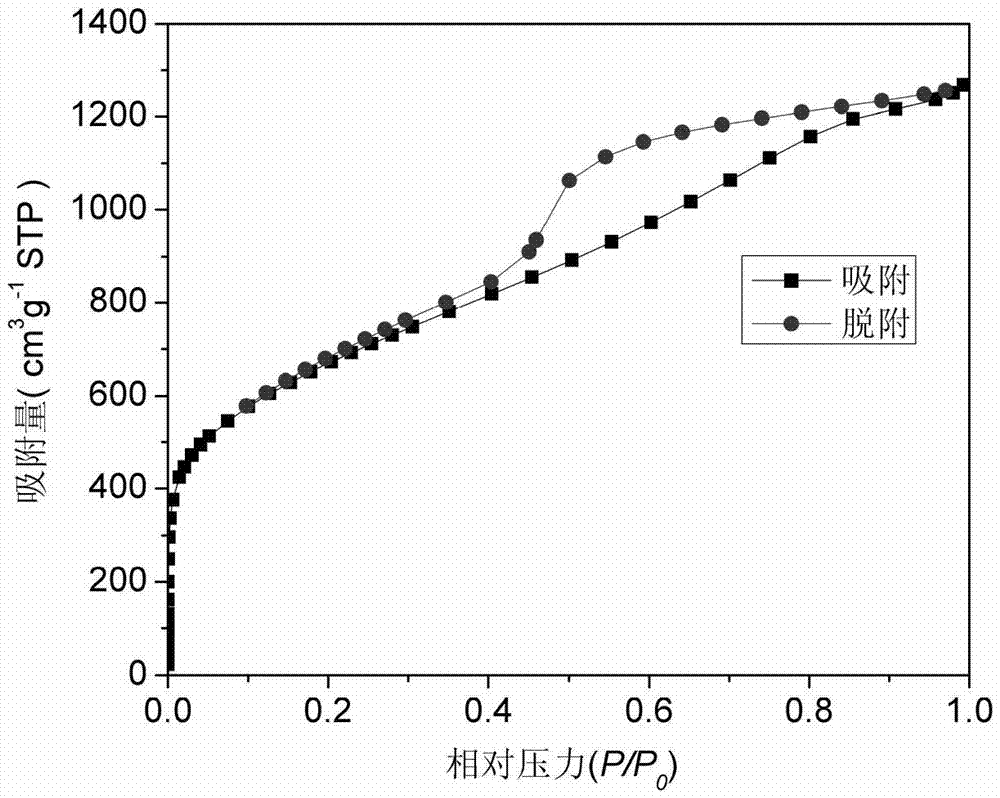
[0034] Weigh 5g of chitosan (purchased from Sinopharm Chemical Reagent Co., Ltd.), fully mix it with 50g of zinc chloride (purchased from Shanghai Xinbao Fine Chemical Factory), place it in a tube furnace, and heat it at 600°C under a nitrogen atmosphere. Calcined for 2h to obtain porous carbon-ZnCl 2 Complex. Then the obtained porous carbon-ZnCl 2 Complex with 1mol L -1 hydrochloric acid treatment at 60 ° C for 2 hours to obtain wet porous carbon, ZnCl obtained after treatment 2 The hydrochloric acid solution can be concentrated and dried to obtain ZnCl 2 Solids are recycled. Finally, the wet porous carbon was dried under vacuum at 100°C for 2 h to obtain porous, figure 1 , 2 shows its microscopic morphology is loose porous structure. The adsorption-desorption curve of porous carbon was obtained by using the method of nitrogen adsorption-desorption test. image 3 As shown, the analysis shows that its specific sur...
Embodiment 2
[0036] Embodiment 2: the preparation of porous carbon
[0037] Weigh 5 g of glucose (purchased from Sinopharm Chemical Reagent Co., Ltd.), fully mix it with 35 g of zinc chloride (purchased from Shanghai Xinbao Fine Chemical Factory), place it in a tube furnace, and calcinate at 700 °C for 10 h under nitrogen atmosphere , to obtain porous carbon-ZnCl 2 Complex. Then the obtained porous carbon-ZnCl 2 Complex with 6mol L -1 Hydrochloric acid was treated at 25°C for 10h to obtain wet porous carbon, and finally, the wet porous carbon was dried at 80°C in nitrogen for 10h to obtain porous carbon. Figure 8 The microscopic morphology is shown as a loose porous structure. The adsorption-desorption curve of porous carbon was obtained by using the method of nitrogen adsorption-desorption test, such as Figure 9 As shown, its specific surface area is 2120m 2 g -1 . The porous carbon contains hierarchical pores with a pore size ranging from 3.2 to 50 nm, such as Figure 10 shown...
Embodiment 3
[0038] Embodiment 3: the preparation of porous carbon
[0039] Weigh 5g of polyacrylonitrile (purchased from Sigma-Aldrich), fully mix it with 25g of zinc chloride (purchased from Shanghai Xinbao Fine Chemical Factory), place in a tube furnace, and calcinate at 400°C for 2h under nitrogen atmosphere , to obtain porous carbon-ZnCl 2 Complex. Then the obtained porous carbon-ZnCl 2 Complex with 0.5mol L-1 The hydrochloric acid was treated at 10° C. for 5 h to obtain wet porous carbon, and finally, the wet porous carbon was dried in argon at 200° C. for 0.5 h to obtain porous carbon. Figure 11 The microscopic morphology is also shown as a loose porous structure. Figure 12 Using the method of nitrogen adsorption and desorption test, the adsorption and desorption curve of porous carbon is obtained, and its specific surface is 670m 2 g -1 . The pore size distribution is mainly multi-level pore size of 0.5-3.72nm, such as Figure 13 shown. When this material is applied to a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com