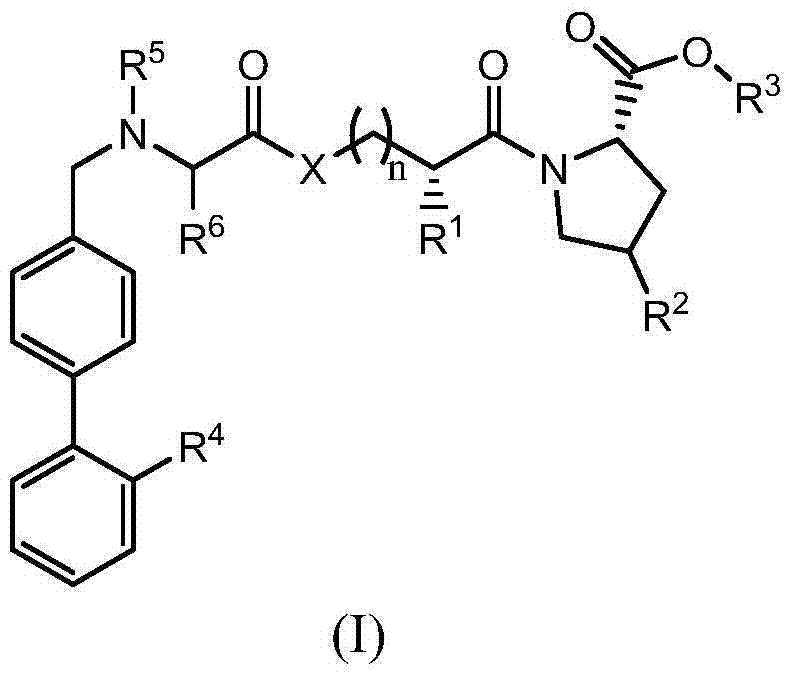
Compound for renin-angiotensin-aldosterone system dual inhibitor
A compound, representative technology, applied in the compound field of dual inhibitors of renin-angiotensin-aldosterone system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
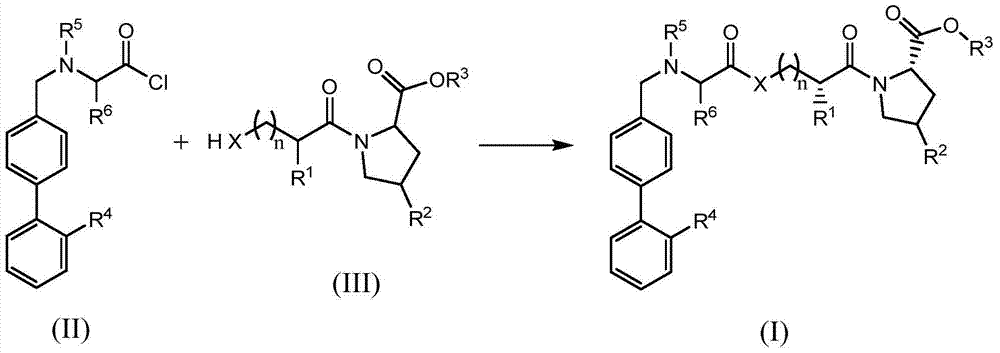
[0039] The preparation of formula (II) compound
[0040] 1. N-pentanoyl-N-(4-(2-(1-trityl-1H-tetrazol-5-yl)phenyl)phenyl)methyl-L-valyl chloride (intermediate 1) Preparation
[0041]
[0042] Step 1, the preparation of N-(4-(2-(1-trityl-1H-tetrazol-5-yl)phenyl)phenyl)methyl-L-valine
[0043] Add potassium carbonate (12.5g) and acetonitrile (85mL) to a 250mL three-necked flask, add L-valine (5.0g), stir, and react for 0.5h; add 2-N-trityl-5-(4' -Bromomethylbiphenyl-2-yl)tetrazolium (16.7g), heated to reflux for 8h, cooled to room temperature, filtered, washed filter cake with 10mL acetonitrile; combined filtrates, rotary evaporation under reduced pressure, recovered solvent. 100 mL of ethyl acetate was added to the residue, and the ethyl acetate layer was washed with brine (30 mL×2). The ethyl acetate layer was dried over anhydrous sodium sulfate, filtered, and the solvent was recovered to obtain the title compound. Molecular formula: C 38 h 35 N 5 o 2 , mass spectro...
Embodiment 1
[0090] (3-((N-(4-(2-(1H-tetrazol-5-yl)phenyl)phenyl)methyl)-N-pentanoyl-L-valyl)thio)-2 -Preparation of -methylpropionyl)-L-proline (compound 1)
[0091]
[0092] Add Intermediate 5 (3.0mmol), Potassium Carbonate (3.0mmol) and DMF (100ml) to the four-neck flask, stir to dissolve, lower the temperature to 0°C, start to add Intermediate 1 (3.0mmol) dropwise, and simultaneously use potassium carbonate The solution maintains the pH of the reaction system at 7.0-8.0, and the temperature is maintained at 0°C. After the dropwise addition is completed, continue to drop the potassium carbonate solution to adjust the pH of the reaction system to 7 until it remains unchanged. The reaction was stirred at room temperature for 3 hours, then water (40 ml) was added thereto, extracted with ethyl acetate, and the solvent was recovered. The residue was adsorbed on silica gel and eluted with n-hexane / acetone to obtain the title compound.
[0093] Molecular formula: C 33 h 42 N 6 o 5 S, ...
Embodiment 2
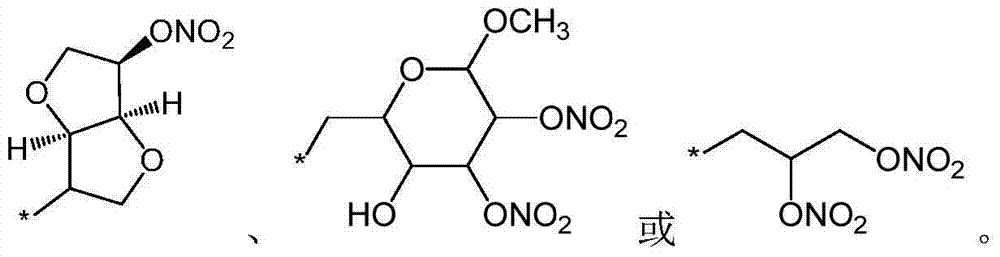
[0097] (3aR,6aS)-3-(N-((2'-(1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-N-pentanoyl Preparation of -L-leucyl-L-alanyl-L-prolyloxy)-6-(nitroxy)hexahydrofuro[3,2-b]furan (compound 2)
[0098]
[0099] In a similar manner to Example 1, intermediate 2 and intermediate 7 were reacted in the presence of basic reagents such as potassium carbonate to obtain the title compound. Molecular formula: C 39 h 50 N 8 o 10 ; Mass spectral analysis (m / z): 790.36 (100.0%), 791.37 (43.1%); Elemental analysis: C, 59.23; H, 6.37; N, 14.17; O, 20.23.
[0100] 1 H-NMR (CDCl 3 ): 8.32(1H), 7.96(2H), 7.60(2H), 7.41-7.47(4H), 6.33(1H), 5.22(1H), 4.64(1H), 4.46(2H), 4.44(1H), 4.29 (1H), 4.14(4H), 3.85(3H), 3.46(2H), 2.30(2H), 1.97-2.05(4H), 1.76(2H), 1.38-1.53(8H), 0.93(9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com