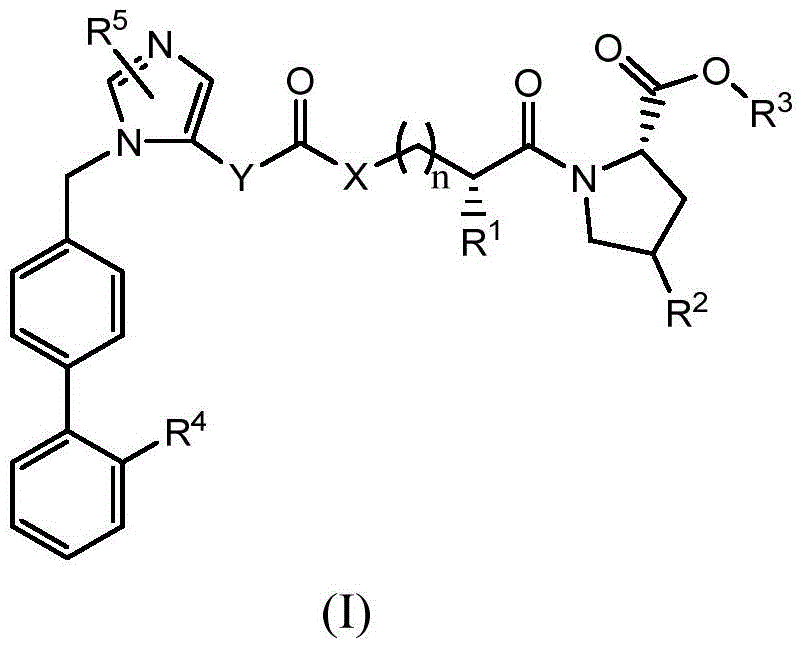
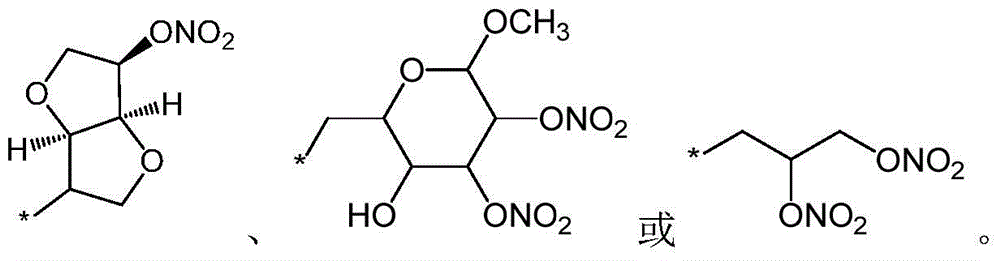
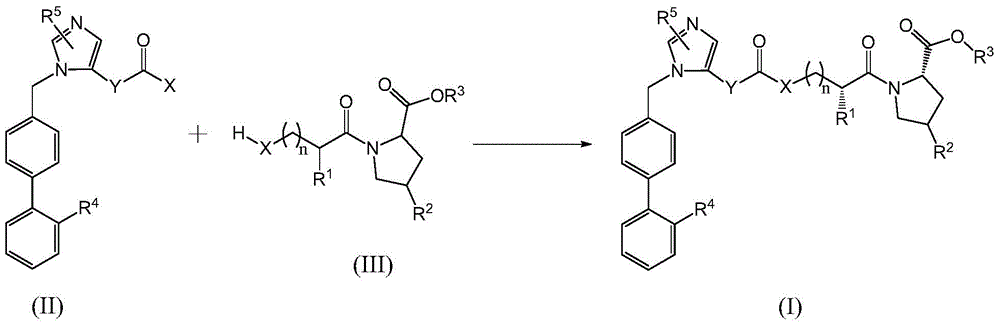
A compound as a dual inhibitor of raas system
A technology of compounds and drugs, applied in the field of compounds as dual inhibitors of RAAS system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The preparation of formula (II) compound
[0038] 1.(2-Butyl-4-chloro-1-((2'-(1-trityl-1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl ) methyl)-1H-imidazole-5-yl)methanol chloroformate (intermediate 1) preparation
[0039]
[0040] Slowly drop the mixed solution of losartan and triethylamine into the dichloromethane solution of solid phosgene (the molar ratio of losartan and solid phosgene is 1:0.5) at low temperature. React at 10 °C for 1 hour. After the reaction is completed, slowly add a certain amount of water dropwise to the reaction solution to decompose the unreacted solid phosgene, and wash with water until the pH value is 5-6. Triethylamine was then added to the reaction mixture, stirred to mix, a solution of trityl chloride in dichloromethane was added, and the resulting reaction mixture was stirred at room temperature. The reaction mixture was washed with water, dried over magnesium sulfate, filtered, and distilled under reduced pressure to obtain the title com...
Embodiment 1
[0064] (3aS,6aR)-6-(nitrooxy)hexahydrofuro[3,2-b]furan-3-yl (((1-((2'-(1H-tetrazol-5-yl)- [1,1'-biphenyl]-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methoxy)carbonyl)-L-alanyl-L- Preparation of proline ester (compound 1)
[0065]
[0066] Add Intermediate 5 (3.0mmol), Potassium Carbonate (3.0mmol) and DMF (100ml) to the four-neck flask, stir to dissolve, lower the temperature to 0°C, start to add Intermediate 1 (3.0mmol) dropwise, and simultaneously use potassium carbonate The solution maintains the pH of the reaction system at 7.0-8.0, and the temperature is maintained at 0°C. After the dropwise addition is completed, continue to drop the potassium carbonate solution to adjust the pH of the reaction system to 7 until it remains unchanged. The reaction was stirred at room temperature for 3 hours, then water was added thereto, extracted with ethyl acetate, and the solvent was recovered. The residue was adsorbed on silica gel and eluted with n-hexane / acetone to obtain t...
Embodiment 2
[0069] (3aS,6aR)-6-(nitrooxy)hexahydrofuro[3,2-b]furan-3-yl ((1-((2'-(1H-tetrazol-5-yl)-[ 1,1'-biphenyl]-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)formyl)-L-alanyl-L-proline ester (Compound 2) Preparation
[0070]
[0071] Add Intermediate 5 (3.0mmol), Potassium Carbonate (3.0mmol) and DMF (100ml) to the four-necked flask, stir to dissolve, lower the temperature to 0°C, start to add Intermediate 2 (3.0mmol) dropwise, and simultaneously use Potassium Carbonate The solution maintains the pH of the reaction system at 7.0-8.0, and the temperature is maintained at 0°C. After the dropwise addition is completed, continue to drop the potassium carbonate solution to adjust the pH of the reaction system to 7 until it remains unchanged. The reaction was stirred at room temperature for 2.5 hours, then water was added thereto, extracted with ethyl acetate, and the solvent was recovered. The residue was adsorbed on silica gel and eluted with n-hexane / acetone to obtain the title co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com