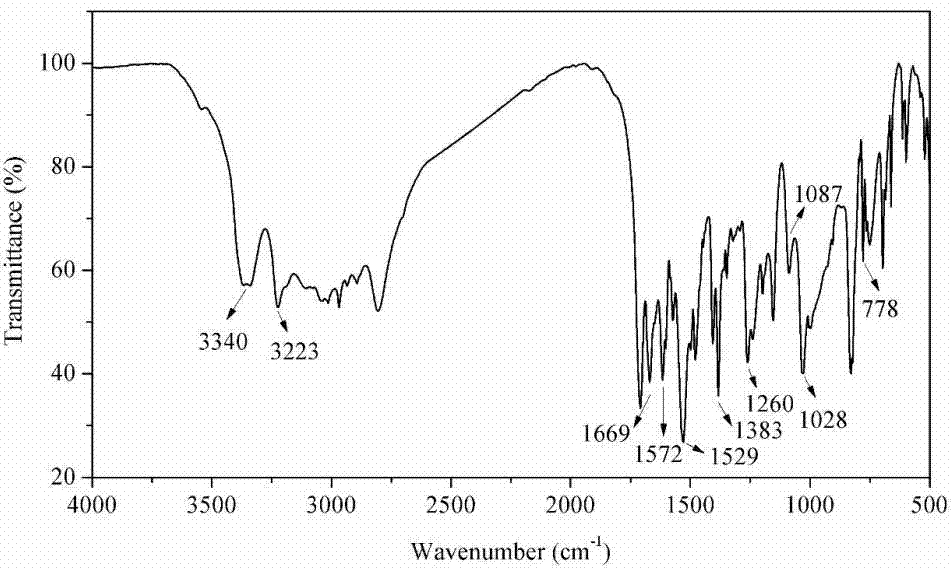
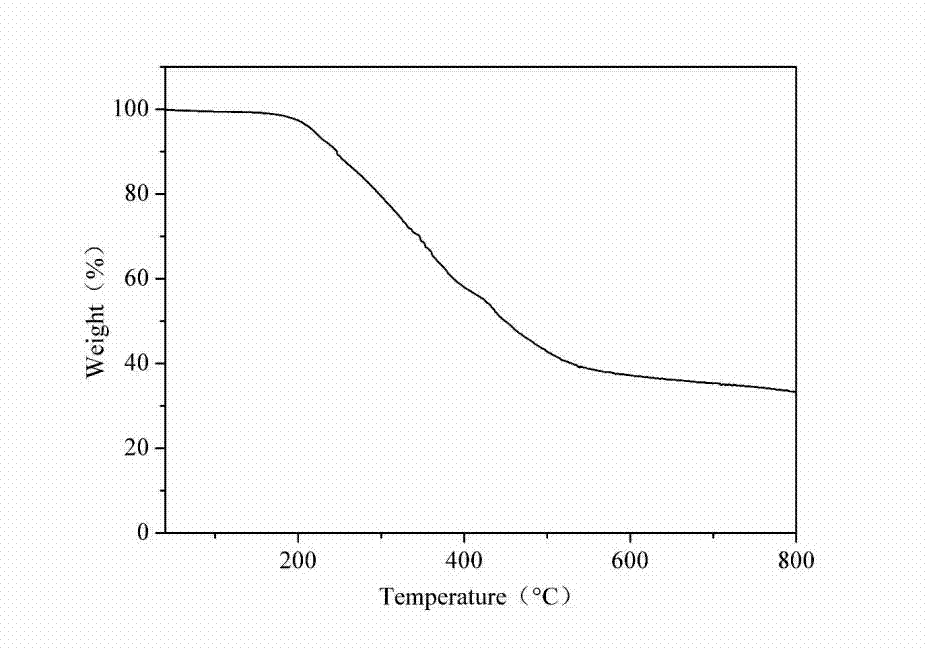
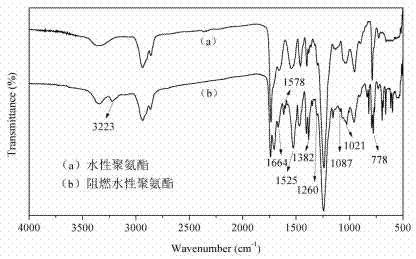
Reactive type intumescent flame retardant for water-based polyurethane and preparation method of reactive type intumescent flame retardant
An intumescent flame retardant and water-based polyurethane technology, which is applied in the field of fine chemicals, can solve the problems of low content, high functionality that makes the polymerization process difficult to control, and the influence of thermodynamic properties of polyurethane coating materials. It achieves good thermal stability, Easy to control thermodynamic properties and excellent storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Add 34.04g (0.25mol) of pentaerythritol and 230.0g (1.50mol) of phosphorus oxychloride to a 250ml four-neck flask equipped with a mechanical stirrer, a thermometer, and a nitrogen condensing reflux tube, heat in an oil bath, and reflux for reaction. React at 60°C for 4h, then heat up to 100°C for 6h. After distilling off unreacted phosphorus oxychloride under reduced pressure, cool, filter, wash with dichloromethane, filter, and vacuum dry at 70°C to obtain white powdered pentaerythritol diphosphate diphosphoryl chloride with a yield of 83%;
[0043] (2) Add 14.85g (0.05mol) of pentaerythritol diphosphate diphosphoryl chloride and 16.94 (0.12mol) of 2,4-diamino- For 6-methoxy-1,3,5-triazine, add 100ml of acetonitrile, stir, dissolve, add 7.91g (0.10mol) of pyridine, heat in an oil bath, and reflux for reaction. Slowly raise the temperature to 80°C and react for 8h. Cool, filter, wash with acetone, and vacuum dry at 70°C to obtain a white powdery reactive intumesce...
Embodiment 2
[0045] (1) Add 34.04g (0.25mol) of pentaerythritol and 211.6g (1.38mol) of phosphorus oxychloride to a 250ml four-necked flask equipped with a mechanical stirrer, a thermometer, and a nitrogen condensing reflux tube, heat in an oil bath, and reflux for reaction. React at 80°C for 2h, then raise the temperature to 110°C for 4h. After the unreacted phosphorus oxychloride was distilled off under reduced pressure, cooled, filtered, washed with dichloromethane, filtered, and vacuum-dried at 80°C to obtain white powdered pentaerythritol diphosphate diphosphoryl chloride, the yield was 82%;
[0046] (2) Add 14.85g (0.05mol) of pentaerythritol diphosphate diphosphoryl chloride and 12.51 (0.10mol) of 2,4-diamino- 6-methyl-1,3,5-triazine, add 100ml N,N - Dimethylformamide, stirred, dissolved, added 15.18g (0.15mol) triethylamine, heated in an oil bath, reflux reaction. Slowly raise the temperature to 90°C and react for 6h. Cool, filter, wash with methyl ethyl ketone and dichlorometha...
Embodiment 3
[0048] (1) Add 34.04g (0.25mol) of pentaerythritol and 214.7g (1.40mol) of phosphorus oxychloride to a 250ml four-neck flask equipped with a mechanical stirrer, a thermometer, and a nitrogen condensing reflux tube, heat in an oil bath, and reflux for reaction. React at 80°C for 2h, then heat up to 105°C for 6h. After distilling off the unreacted phosphorus oxychloride under reduced pressure, cool, filter, wash with dichloromethane, filter, and vacuum dry at 70°C to obtain white powdered pentaerythritol diphosphate diphosphoryl chloride with a yield of 84%;
[0049] (2) Add 14.85g (0.05mol) of pentaerythritol diphosphate diphosphoryl chloride and 17.79 (0.14mol) of 4,6-diamino- 2-Hydroxy-1,3,5-triazine, add 100ml N,N -Dimethylacetamide, stirred, dissolved, added 9.02g (0.20mol) dimethylamine, heated in an oil bath, reflux reaction. Slowly raise the temperature to 70°C and react for 8h. Cool, filter, wash with acetone and toluene, and vacuum-dry at 80°C to obtain a white powd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com