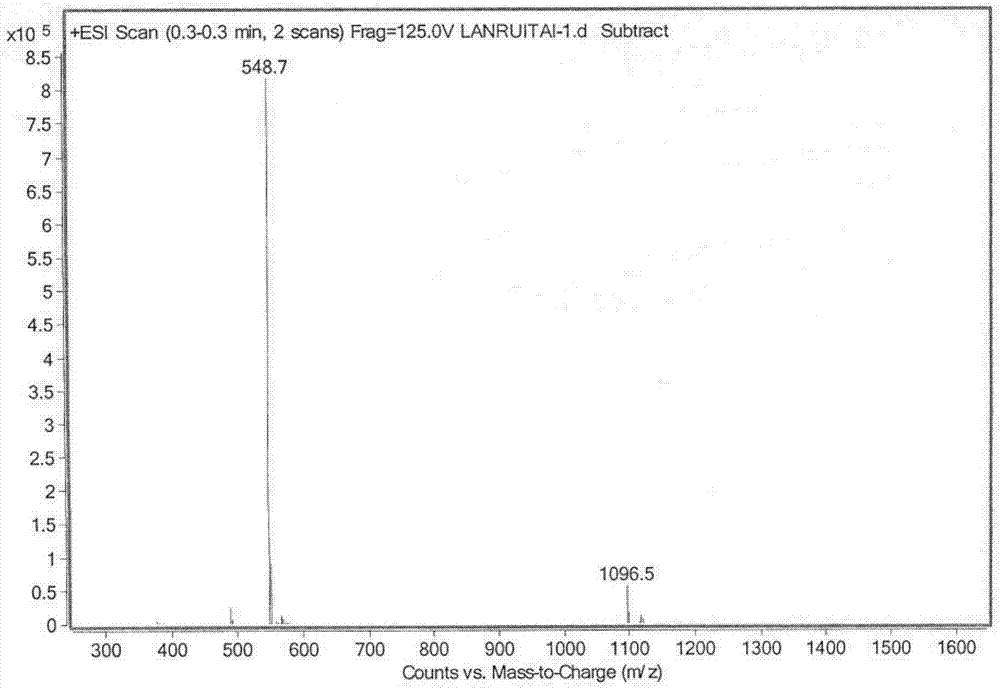
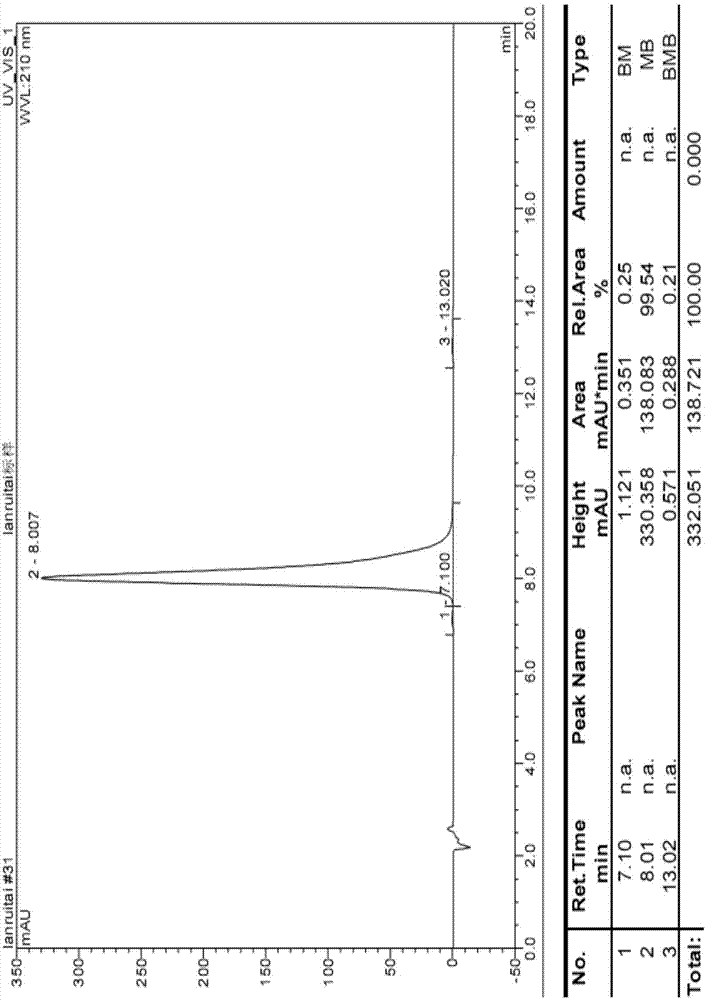
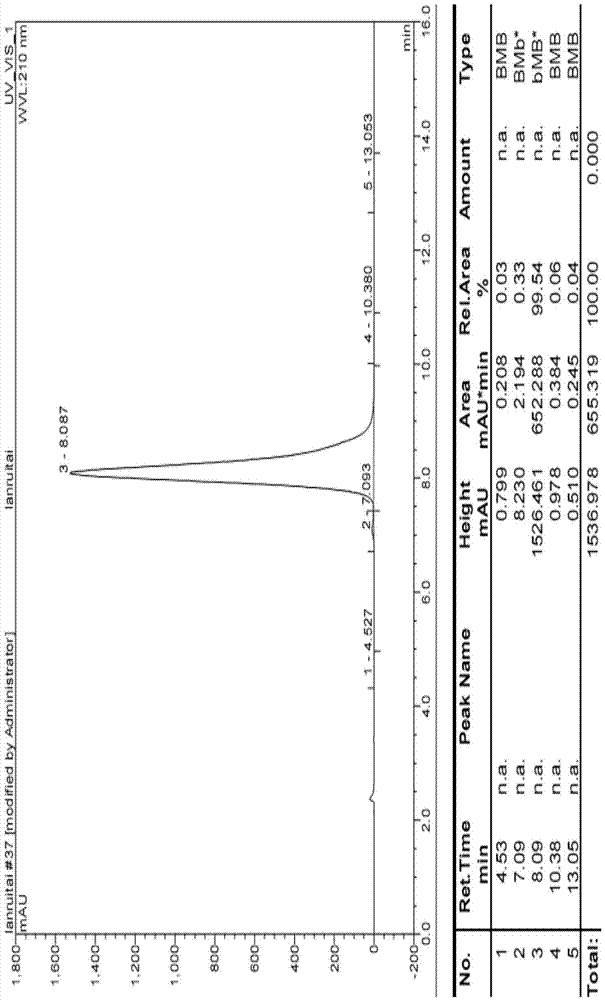
Preparation method for lanreotide
A technology of lanreotide and peptide resin, which is applied in the field of peptides and can solve the problems of high cost and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] The invention discloses a preparation method of lanreotide, and those skilled in the art can learn from the content of this article and appropriately improve the process parameters to realize it. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The method and application of the present invention have been described through preferred embodiments, and the relevant personnel can obviously make changes or appropriate changes and combinations to the method and application described herein without departing from the content, spirit and scope of the present invention to realize and Apply the technology of the present invention.
[0063] The raw materials used in the preparation method of lanreotide provided by the present invention are all available in the market. Among them, the amino acid was purchased from Jill Biochemical Sha...
Embodiment 1
[0067] The preparation of embodiment 1 lanreotide peptide resin
[0068] 1. Preparation of Fragment Boc-Nal-Cys(Trt)-OH
[0069] Dissolve Boc-3-(2-naphthyl)-D-alanine in dimethylformamide, add excess HOSU and excess DCC, react at 10-15°C for 2-3 hours after dissolving, and detect Boc -3-(2-naphthyl)-D-alanine reacted completely, and filtered to obtain the filtrate; the filtrate was added dropwise to the H-Cys(Trt)-OH solution (sodium carbonate and H-Cys(Trt)-OH were added to the water prepared), reacted at 10-15°C for 10-15 hours, detected that Boc-3-(2-naphthyl)-(D)Phe-OSU was completely reacted, adjusted the pH value of the solution to 3.0-4.0 with acetic acid, filtered, and added acetic acid Ethyl extraction, the ethyl acetate layer was washed with saturated sodium chloride solution for 3-5 times, then dried with anhydrous sodium sulfate for 3 hours, filtered and the filtrate was distilled under reduced pressure to remove most of the ethyl acetate, then crystallized with p...
Embodiment 2
[0100] Embodiment 2 Preparation of lanreotide crude refined peptide
[0101] Preparation of crude lanreotide Nal-Cys-Tyr-D-Trp-Lys-Val-Cys-Thr-NH2
[0102] (1) Prepare the lysate: Add trifluoroacetic acid (TFA) / phenol / 1,2-ethanedithiol (EDT) / triisopropylsilane (Tis) in a volume ratio of 89:3:3:5 to the glass Or refrigerate in a PTFE container for 2 hours.
[0103] (2) Excision of resin and other amino acid side chain protecting groups: Take out the pre-cooled lysate, add 8-12ml of peptide cutting solution to 1g of lanreotide peptide resin prepared in Example 1, and put the above-mentioned fragment condensation peptide resin in Add to the lysate under stirring, react at room temperature for 2 to 2.5 hours, then filter, add the filtrate to pre-cooled ether, stir and precipitate, centrifuge the precipitate, remove the supernatant, wash the precipitate with ether four times, and dry in vacuum to obtain a dry powder 6.2g, which is the crude straight chain lanreotide.
[0104] (3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com