A strain of Haemophilus parasuis serotype 13 and its application
A technology of Haemophilus suis and Haemophilus suis disease, applied in the field of Haemophilus parasuis type 13, can solve the problems of farmers increasing the dosage of antibiotics, pig products exceeding the standard of antibiotics, and poor treatment effect, etc., and achieve biological Stable performance, strong pathogenicity, and the effect of reducing economic losses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Isolation and identification of serotype 13 Haemophilus parasuis
[0035] 1. Isolation of pathogenic bacteria
[0036] Use a sterile inoculation loop to take samples from fresh lungs, pericardial effusion, joint fluid and other disease materials of suspected sick pigs, inoculate them by streaking on TSA medium (containing final concentration of 5% serum and 0.001% NAD), and culture at 37°C After 24h to 48h, the growth was observed, and Gram staining was performed on suspected colonies. Pick a single colony, adopt the method of partitioning and marking on the TSA solid medium, and carry out 3 to 5 times of single colony purification and culture until there is no miscellaneous bacteria.
[0037] 2. Identification of pathogenic bacteria
[0038] 2.1 NAD dependence test
[0039] In a Class II biological safety cabinet, use an inoculation loop to pick up a single colony of the above-mentioned suspicious bacteria, and streak horizontally on a NAD-free TSA plate. ...
Embodiment 2
[0058] Embodiment 2 white oil adjuvant inactivated vaccine preparation
[0059] 1. Antigen preparation
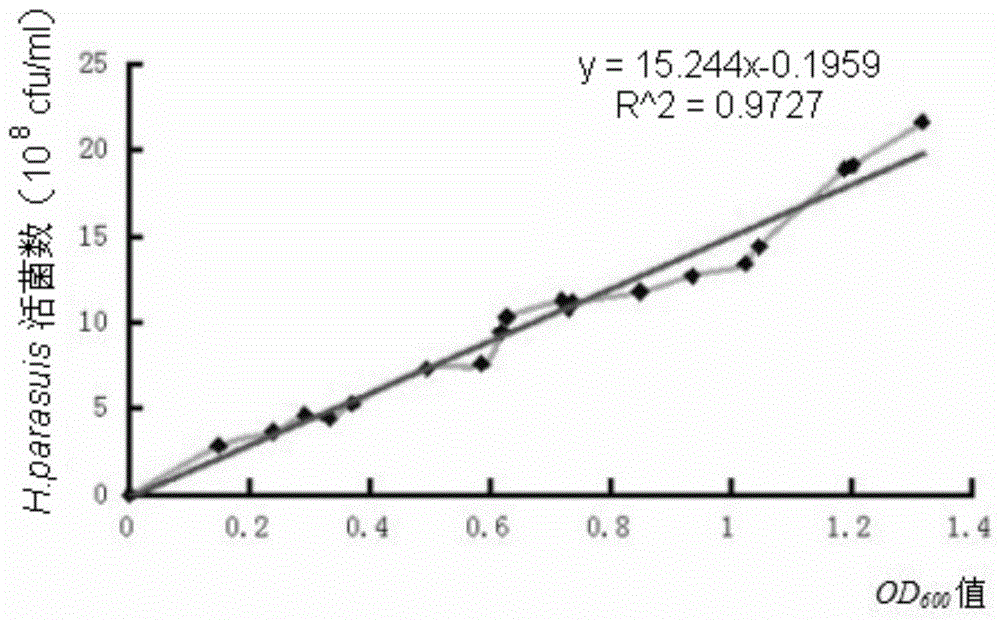
[0060] Inoculate Haemophilus parasuis GX0905 strain on TSA solid medium for resuscitation, pass three generations continuously for rejuvenation, inoculate the rejuvenated Haemophilus parasuis in TSB liquid medium, and culture in a shaker at 37°C for about 12h to 14h , as the seed liquid, then inoculate the seed liquid into TSB liquid medium preheated to 37°C according to 1% of the total volume of the medium, 37°C, 180r / min shaking and expanding culture for 14h-16h, and measure the bacterial liquid with a spectrophotometer OD 600 Value, according to the number of viable bacteria and OD 600 Value correlation standard curve formula to calculate the total bacterial content. Then add formaldehyde with a final concentration of 0.2% to the bacterial solution to inactivate the bacteria, place in a 37°C incubator to inactivate for 14h-16h, and shake once every 4h-5h during the in...
Embodiment 3
[0063] Embodiment 3 preparation of aluminum gel adjuvant inactivated vaccine
[0064] Antigen preparation steps are as in Example 2.
[0065] Vaccine preparation:
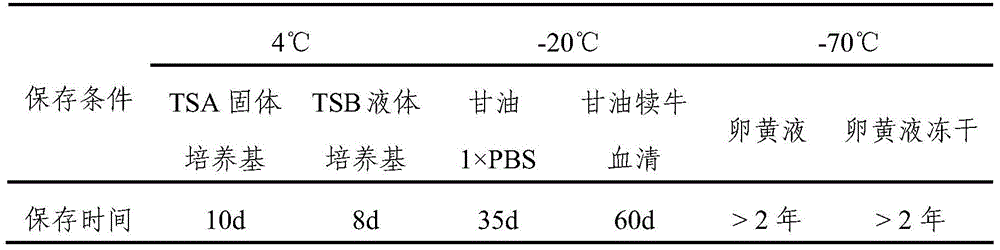
[0066] Evenly mix the bacteria solution that has passed the inactivation test with 20% sterile aluminum hydroxide saline dilution solution, let it stand for precipitation at room temperature for 24 hours, suck out the supernatant according to 1 / 2 of the total volume, and then add Thimerosal solution with a final content of 1 / 10,000. The content of bacteria in the finished vaccine is 2.5×10 9 CFU / mL CFU / mL, store at 4°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com