Week-acting transdermal pramipexole patch and preparation method thereof
A technology of pramipexole and transdermal patch, which is applied to medical preparations with no active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve the problems of poor skin penetration and poor fat solubility. Achieve the effects of small hair follicle density, strong lipophilicity, and convenience for pharmacokinetic experiments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Polyvinyl alcohol (PVA17-88) 1.5g, polyvinylpyrrolidone (PVP) 0.3g, propylene glycol 0.2g, glycerol 0.2g, ethylenediaminetetraacetic acid disodium 0.1g, pramipexole 40mg to prepare pramipexole paste sheet.
[0041] Polyvinyl alcohol (PVA17-88) 1.5g, polyvinylpyrrolidone (PVP) 0.3g, propylene glycol 0.2g, glycerol 0.2g, disodium edetate 0.1g, pramipexole hydrochloride 40mg, to prepare pramipexole hydrochloride paste sheet.
[0042] Preparation method: Fully swell polyvinyl alcohol and polyvinylpyrrolidone with 60% ethanol, add propylene glycol and glycerin, stir under a water bath at 70°C with magnetic stirring to fully dissolve and mix, add the drug solution when the temperature is lowered to 40°C, mix well, and let stand When the bubbles disappear, the film is laid by the casting method, and the composite is dried after forming and cooling, covering the polyethylene-aluminum-polyethylene composite film and the polyethylene anti-adhesive layer, and the divided dose is prepa...
Embodiment 2
[0045] Polyvinyl alcohol (PVA17-88) 1.6g, polyvinylpyrrolidone (PVP) 0.4g, eucalyptus oil 0.2g, glycerin 0.1g, ethylenediaminetetraacetic acid disodium 0.1g, pramipexole 100mg, to prepare pramipexole Agent.
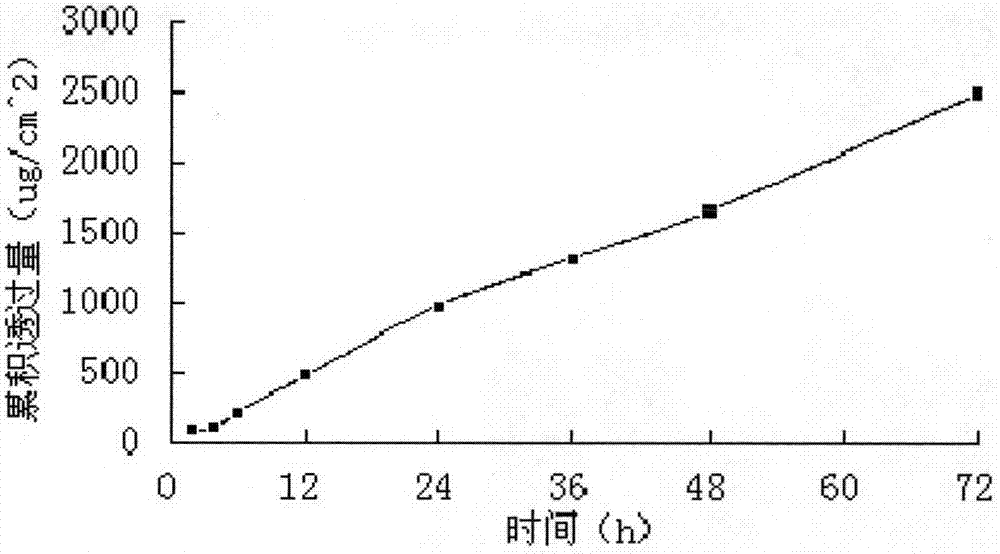
[0046] Preparation method: Fully swell polyvinyl alcohol and polyvinylpyrrolidone with 60% ethanol, add glycerin and penetration enhancer, and stir under a water bath at 70°C with magnetic stirring to fully dissolve and mix. When the temperature is reduced to 40°C, add the drug solution and mix evenly. Leave it to stand until the bubbles disappear, spread the film by casting method, dry, form and cool and then compound, cover the polyethylene-aluminum-polyethylene composite film and the polyethylene anti-sticking layer, and prepare the divided dose to contain pramipexole 10.0mg / cm 2 Of the patch.
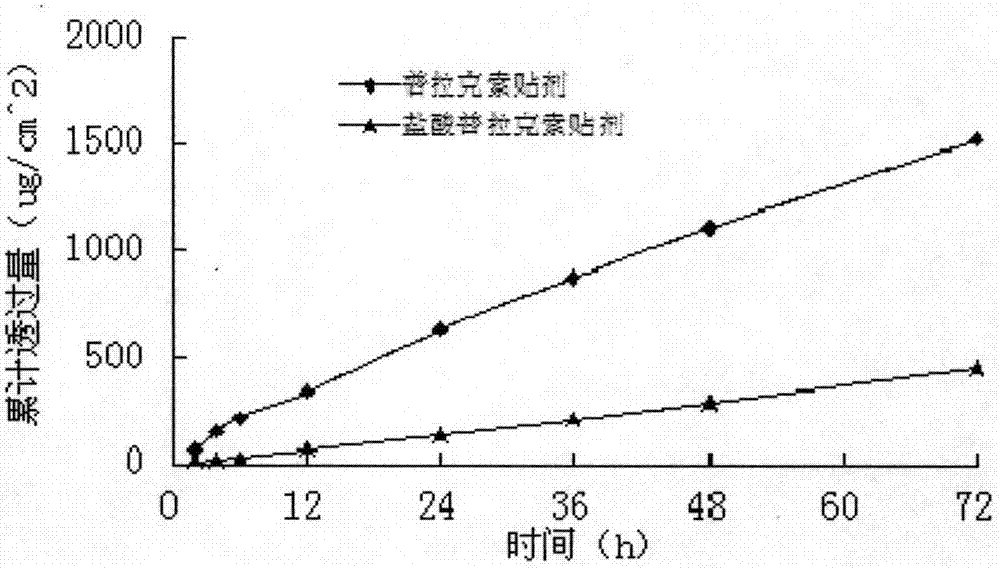
[0047] A modified Franz diffusion cell was used to measure the permeability of the transdermal patch on the inner skin of rabbit ears. The patch area used in the transdermal diffusi...
Embodiment 3
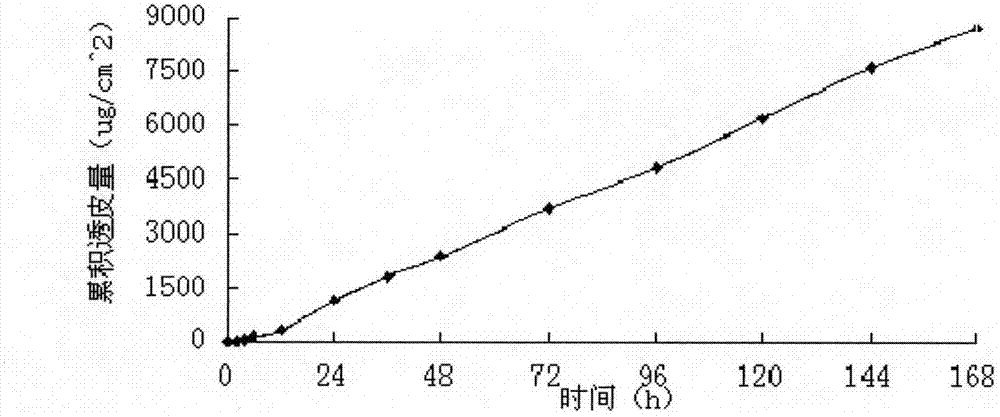
[0049] The first layer (one layer close to the release layer) composition: polyvinyl alcohol (PVA17-88) 2.8g, polyvinylpyrrolidone (PVP) 0.4g, eucalyptus oil 0.3g, propylene glycol 0.2g, glycerol 0.3g, ethylene dichloride Disodium aminetetraacetic acid 0.2g, pramipexole free base 40mg.
[0050] The second layer (close to the backing layer) composition: polyvinyl alcohol (PVA17-88) 1.6g, polyvinylpyrrolidone (PVP) 0.5g, eucalyptus oil 0.4g, propylene glycol 0.1g, glycerin 0.2g, ethylene diamine tetra Disodium acetate 0.1g, pramipexole free base 120mg.
[0051] Preparation method: Fully swell polyvinyl alcohol and polyvinylpyrrolidone with 60% ethanol, add propylene glycol and glycerin, stir under a water bath at 70°C with magnetic force to fully dissolve and mix. When the temperature is lowered to 40°C, add the drug and penetration enhancer solution and mix well. After that, let it stand until the bubbles disappear, cast the film, dry, form and cool and composite, cover the polyeth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Permeation rate | aaaaa | aaaaa |
| Permeation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com