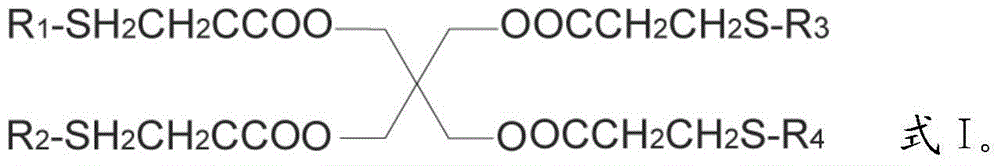
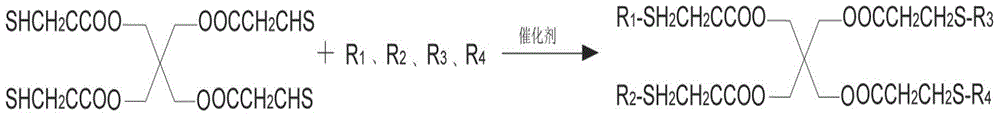
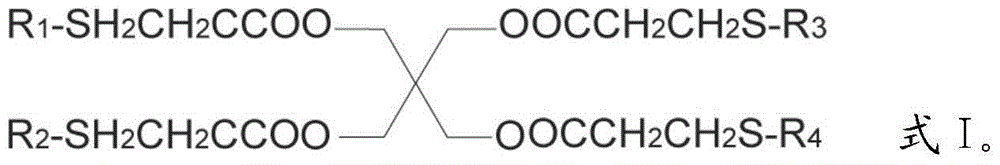
Preparation method for pentaerythritol tetra (3-R-alkyl thiopropionic acid)
A technology of alkylthiopropionic acid and pentaerythritol ester, which is applied in the preparation of thioether and organic chemistry, can solve the problems of toxicity and difficulty in obtaining alkylthiol, and achieve high activity, improved structural activity, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Put 244g of pentaerythritol tetra-3-mercaptopropionate and 7.3g of potassium tert-butoxide into a 1000ml reactor, turn on the nitrogen replacement, turn on the agitator and raise the temperature to 35 degrees, slowly add 450.8g of n-tetradecene and keep the reaction The temperature is 35-40 degrees. After the addition, keep warm at 40 degrees for 5 hours, add 3.91g of acetic acid, after precision filtration, depressurize to -0.01MPa, temperature 290 degrees, recover excess n-tetradecene and trace unreacted pentaerythritol by distillation Tetra-3-mercaptopropionate obtained 596.4g white solid after cooling, the yield was 93.77%, and the product purity was 99.5%.
Embodiment 2
[0028] Put 244g of pentaerythritol tetra-3-mercaptopropionate and 4.88g of sodium methylate into a 1000ml reactor, turn on the nitrogen replacement, turn on the stirrer and heat up to 30 degrees, slowly add 378g of n-dodecene and keep the reaction temperature at 30°C ~35°C, keep warm at 35°C for 6 hours after the addition, add 5.43g of acetic acid, after precision filtration, depressurize to -0.01MPa, temperature 290°C, recover excess n-dodecene and trace unreacted pentaerythritol tetra-3 -Mercaptopropionate, after cooling, 573.1g white solid was obtained, the yield was 98.8%, and the product purity was 99.7%.
Embodiment 3
[0030] Put 244g of pentaerythritol tetra-3-mercaptopropionate and 2.8g of sodium hydroxide into a 1000ml reactor, after opening the nitrogen replacement, open the agitator and heat up to 30 degrees, slowly add 336g of n-decene and keep the reaction temperature at 30°C ~33°C, keep warm at 38°C for 5 hours after the addition, add 4.2g of acetic acid, after precision filtration, depressurize to -0.01MPa, temperature 290°C, recover excess n-decene and trace unreacted pentaerythritol tetra-3- Mercaptopropionate obtained 504.4g of white waxy solid after cooling, the yield was 96.2%, and the product purity was 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com