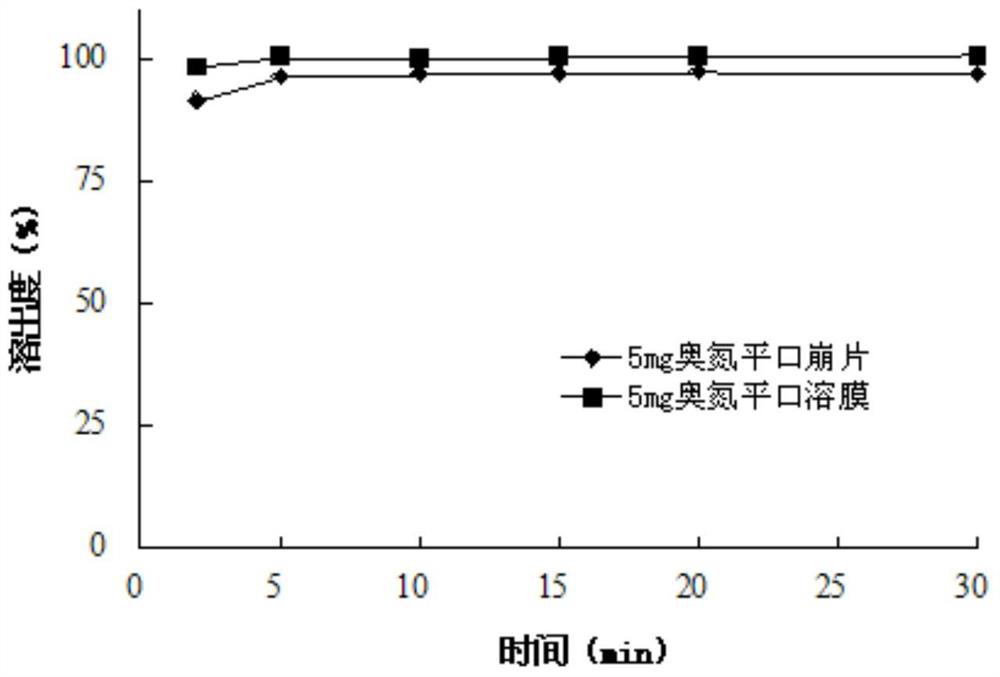
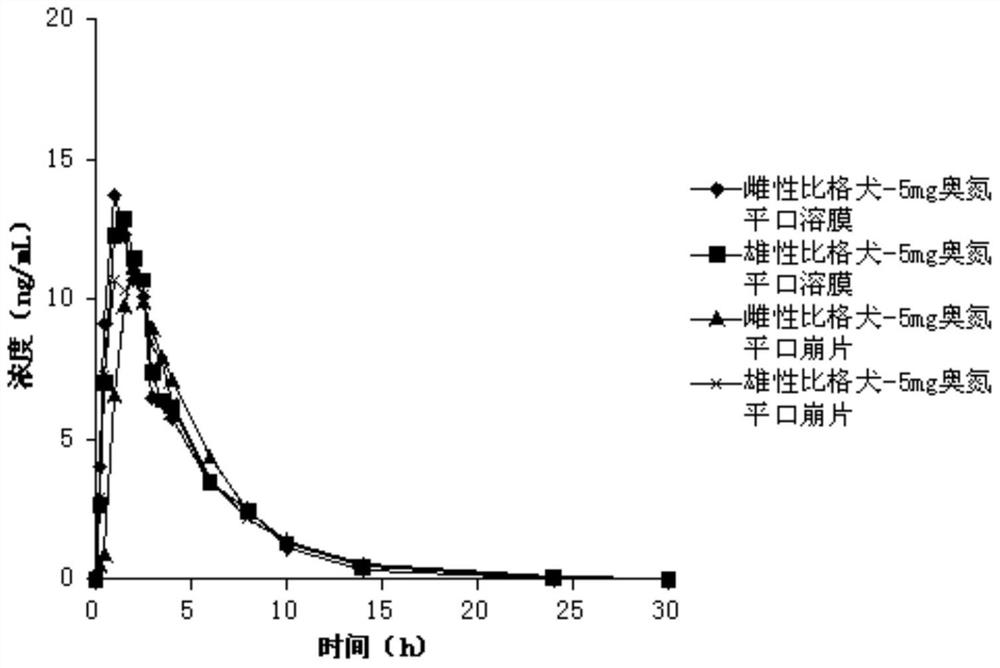
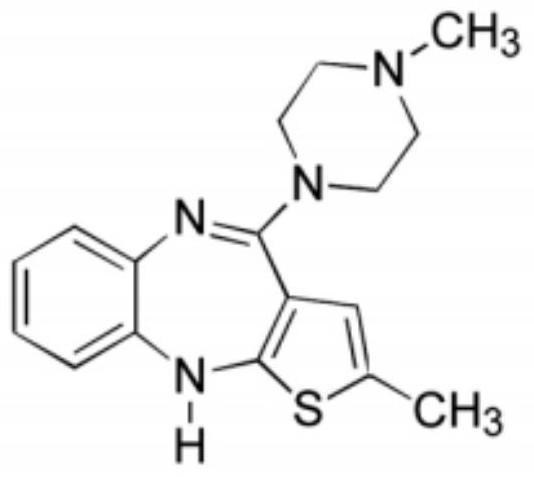
Olanzapine oral instant film
An oral instant film, olanzapine technology, applied in pharmaceutical formulations, nervous system diseases, sheet delivery, etc., can solve the problems of increased production cost, high price of auxiliary materials, and high equipment requirements, and achieves short production cycle and oral absorption. The effect of fast speed and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The prescription quantity is 1000 tablets (5mg specification)
[0041]
[0042] *Used in prescription but removed during processing.
[0043] First add the above-mentioned amount of sucralose, glycerin and polysorbate 80 into the aqueous solution, after stirring and dissolving, add hypromellose (15 centipoise), fully stir and dissolve to obtain the hypromellose blank glue, and then Add olanzapine and stir until evenly dispersed to obtain olanzapine drug-containing glue, stir and defoam under vacuum conditions, apply the drug-containing glue evenly on the polyester tape after defoaming, and heat at 70-80°C Drying, yellow after drying, extremely poor toughness and strength, unable to form a film.
Embodiment 2
[0045] The prescription quantity is 1000 tablets (5mg specification)
[0046]
[0047]
[0048] *Used in prescription but removed during processing.
[0049] First add the above-mentioned amount of sucralose, glycerin and polysorbate 80 into the aqueous solution, stir to dissolve, then add hydroxypropyl cellulose, stir and dissolve fully to obtain a hydroxypropyl cellulose blank glue, then add olanzapine and Stir until uniformly dispersed to obtain olanzapine drug-containing glue, stir and defoam under vacuum conditions, apply the drug-containing glue evenly on the polyester tape with a scraper after degassing, heat and dry at 70-80°C, and after drying, it becomes Yellow, very poor in toughness and strength, unable to form a film.
Embodiment 3
[0051] The prescription quantity is 1000 tablets (5mg specification)
[0052]
[0053] *Used in prescription but removed during processing.
[0054] First add the above-mentioned amount of sucralose, glycerin and polysorbate 80 into the aqueous solution, stir to dissolve, then add polyvinyl alcohol, fully stir and dissolve in a water bath at 80°C to obtain a polyvinyl alcohol blank glue, then add olanzapine and stir Until the olanzapine drug-containing glue is uniformly dispersed, stir and defoam under vacuum conditions, apply the drug-containing glue evenly on the polyester tape with a scraper, heat and dry at 70-80°C, and turn yellow after drying , The toughness and strength are extremely poor, and it is impossible to form a film.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com