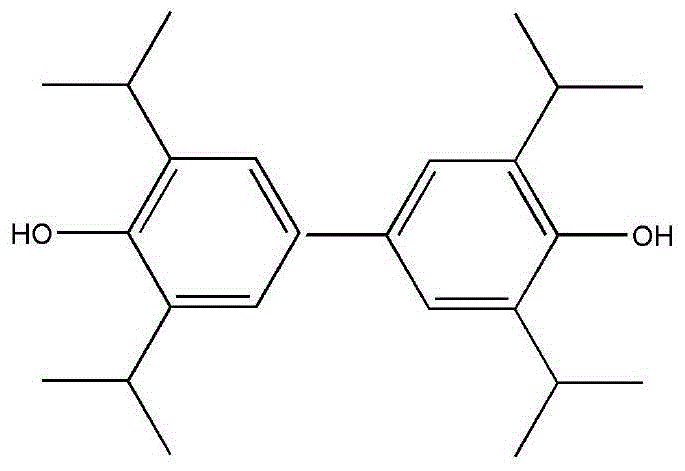
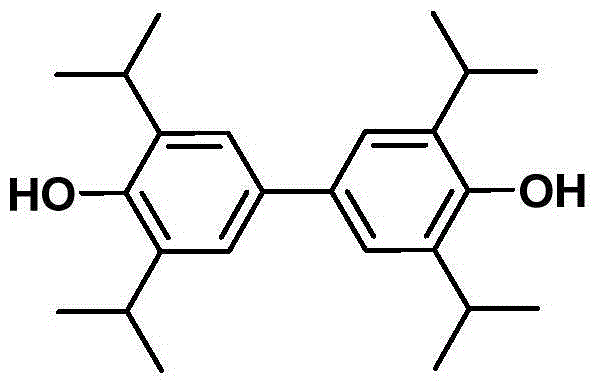
Application of 3,3',5,5'-tetraisopropyl-4,4'-diphenol in prevention and treatment for ischemic stroke
A technology for ischemic stroke and diphenol, applied in the field of medicine, can solve problems such as limiting clinical application, and achieve the effects of less trauma and accurate control of ischemia and reperfusion time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1, 3,3',5,5'-tetraisopropyl-4,4'biphenol
[0062]
[0063]Weigh 20g of propofol and dissolve it in 100ml of ethyl acetate, then add 24.75g of silver carbonate and 10g of anhydrous magnesium sulfate, stir at room temperature for 2h, and detect that the reaction is complete. Add water to the reaction liquid until no bubbles are generated, filter the solid, wash with ethyl acetate, remove the water phase, dry the ethyl phase with anhydrous sodium sulfate for 1 h, filter, evaporate the filtrate to dryness under reduced pressure, add appropriate amount of Washed with water and methanol to obtain 12.30 g of red crystals. Weigh 7 g of the above-mentioned red solid and dissolve it in 100 mL of ethyl acetate. In addition, dissolve 27.66 g of sodium hydrosulfite in 1 mol / L NaOH, mix the two, stir at room temperature for 1.5 h, and detect that the reaction is complete. The ethyl acetate phase was separated, the aqueous phase was extracted twice with ethyl acetate, drie...
Embodiment 2
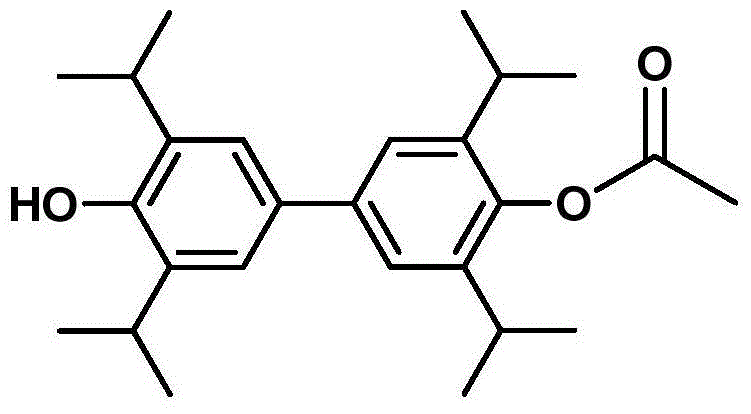
[0065] Example 2, 4'-hydroxyl-3,3',5,5'-tetraisopropylbiphenyl-4-acetate
[0066]
[0067] Dissolve 4'-benzyloxy-3,3',5,5'-tetraisopropylbiphenyl-4-acetate (5 g, 10.27 mmol) in 200 mL of methanol at room temperature, and then add 10% palladium carbon ( 570mg), vacuumize, hydrogen, repeat three times, seal and react at room temperature for 10h, filter the palladium carbon in the reaction solution, and evaporate the filtrate under reduced pressure to obtain 4'-hydroxy-3,3',5,5'-tetraiso Propylbiphenyl-4-acetate (3.9 g, 95.73%), white solid.
[0068] white solid; 1 H NMR (300MHz, CDCl 3 )δ7.19 (s, 4H), 4.86 (s, 1H), 3.37-3.32 (m, 4H), 3.16 (s, 3H), 1.20 (d, 24H).
Embodiment 3
[0069] Example 3, 3,3',5,5'-tetraisopropylbiphenyl-4'4-diacetate
[0070]
[0071] Add 4,4'-dihydroxy-3,3',5,5'-tetraisopropylbiphenyl (5g, 14.10mmol) into 30mL of acetic anhydride, under nitrogen protection, reflux for 3h, the reaction solution Cool to room temperature, remove acetic anhydride under reduced pressure, add water (200 mL) to the residue, a white solid appears, wash with 10% cold ethanol (100 mL) and water (200 mL), dry to give 3,3',5,5' - Tetraisopropylbiphenyl-4'4-diacetate (6 g, 95.06%). white solid.
[0072] white solid, 1 H NMR (300MHz, CDCl 3 )δ7.19(s,4H),2.91-2.89(m,4H),2.32(s,6H),1.19(d,24H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com