Preparation method of room-temperature preserved bifidobacteria enteric microcapsule
A bifidobacteria, room temperature preservation technology, applied in microcapsules, food preparation, medical preparations with inactive ingredients, etc., can solve the problems of reduced recovery rate, increased amount of capsule material, irregular shape of bacteria powder particles, etc. To achieve the effect of improving tolerance and heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1. Preparation method of bifidobacterium NQ-1501 enteric-coated microcapsules preserved at room temperature
[0020] The preparation method of bifidobacterium NQ-1501 enteric-coated microcapsules which can be stored at room temperature comprises the following steps:
[0021] 1) Prepare an enteric coating liquid composed of 40% polyacrylic acid resin latex, 1% triethyl citrate, 2% talcum powder and 57% purified water for subsequent use;
[0022] 2) Put 1.5kg of Bifidobacterium NQ-1501 bacteria powder in a fluidized bed coating machine, spray 500ml of 30% maltodextrin aqueous solution as a binder into the fluidized bed to prepare stable capsule core granules for later use ;
[0023] 3) Put the capsule core in a fluidized bed coating machine, first use 900ml of 30% starch aqueous solution as the coating liquid for bottom spray coating, and after spraying and drying, the primary microcapsules are obtained. Afterwards, 1875ml of polyacrylic acid resin enteric-coati...
Embodiment 2
[0025] Example 2. Bifidobacterium NQ-1501 enteric-coated microcapsule effect experiment
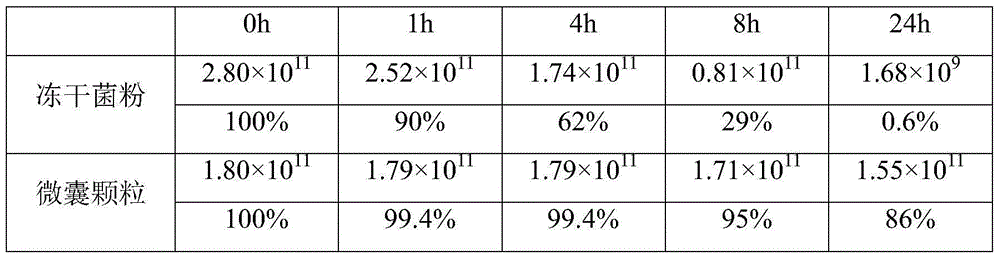
[0026] 1) Heat resistance test
[0027] 10 g of the freeze-dried bifidobacteria powder of Example 1 and the coated microcapsules were each placed in a sealed aluminum-plastic bag, and placed at 50° C. for 1, 4, 8, and 24 hours to determine the number of viable bacteria immediately. The result is as follows:
[0028]
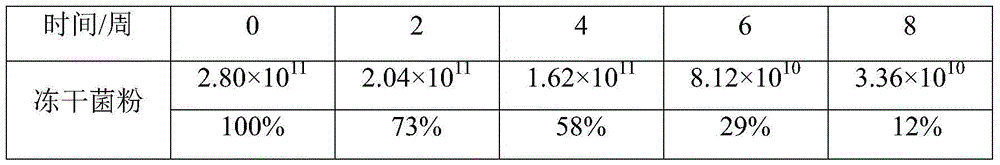
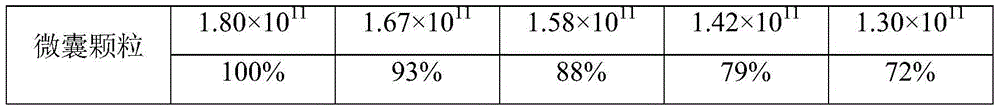
[0029] In Example 1, the freeze-dried bacteria powder of bifidobacteria and the coated microcapsules were packed into sealed aluminum-plastic bags in an amount of 10 g per portion, stored at 25° C. for 8 weeks, and the results of the changes in the number of viable bacteria were as follows:
[0030]
[0031]
[0032] 2) gastric acid resistance test
[0033] Dissolve 1 g of the bifidobacterium freeze-dried powder and coated microcapsules in Example 1 in 100 ml of hydrochloric acid solution with a pH of 1.2, and measure the number of viable bacteria after bathing in...
Embodiment 3
[0036] Example 3. Preparation method of bifidobacterium NQ-1501 enteric-coated microcapsules preserved at room temperature
[0037] Concrete preparation method is identical with the technological process of embodiment 1, difference is as follows:
[0038] 1) Prepare an enteric coating solution composed of 30% polyacrylic acid resin latex, 2% triethyl citrate, 5% talcum powder and 63% purified water for later use.
[0039] 2) Evenly mix the bifidobacterium cells and the freeze-drying protective agent according to the mass ratio of 1:10, then vacuum freeze-dry, crush and pass through a 40-mesh sieve to obtain the bifidobacterium powder, and the number of live bacteria in the powder is 3.10×10 11 cfu / g.
[0040] 3) Put 2.0 kg of bifidobacterium powder in a fluidized bed coating machine, spray 750 ml of 40% maltodextrin aqueous solution as a binder into the fluidized bed to prepare stable capsule core granules for future use.
[0041] 4) Put the capsule core in a fluidized bed c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com