Main outer membrane protein epitope vaccine of chlamydia trachomatis based on HBcAg vector and application of main outer membrane protein epitope vaccine
A major outer membrane protein, Chlamydia trachomatis technology, applied in the fields of biomedical technology and immunology, can solve problems such as unsatisfactory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Embodiment 1. Obtaining and modification of HBcAg carrier gene
[0106] Using the serum samples of hepatitis B virus carriers (Dasanyang) in Wenzhou area as a template, PCR primers were designed and synthesized, and enzyme cutting sites (Ned I and Hind III) were introduced at both ends to amplify the complete open reading frame of HBcAg. The PCR product and The vector pET21a(+) was ligated after double digestion at the same time to construct the recombinant plasmid pET21a(+) / HBcAg(wt), which was identified by sequencing.
[0107] In order to enhance the carrier effect of HBcAg and facilitate the operation of molecular cloning, the HBcAg nucleotide (SEQ ID NO: 3) was modified and designed, that is, the universal Th epitope PADRE was inserted into its C-terminus, and the N- and C-terminals of the HBcAg carrier Each introduces a His-tag sequence to facilitate purification; at the same time, base mutations are performed in its MIR region (78th and 82nd amino acids) to for...
Embodiment 2
[0108] Example 2. Preparation and in vitro expression of HBcAg / CtMOMP epitope vaccine, analysis of VLPs formation
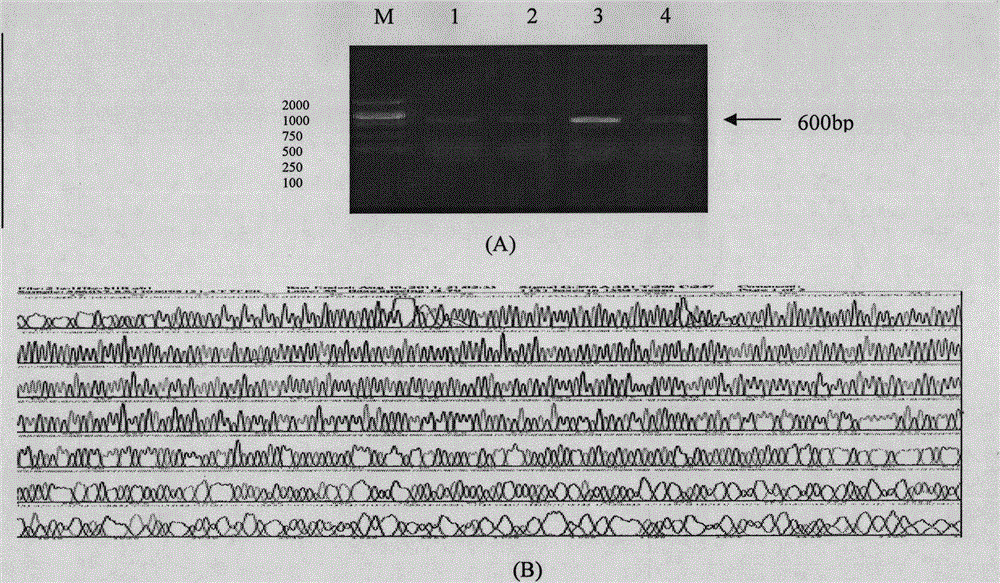
[0109] Based on the prokaryotic codon-optimized Ct MOMP immunodominant epitope gene and tandem epitope gene, complementary primers were designed and synthesized, and the two ends were respectively introduced with restriction sites BamH I and Sac I, and the target gene was obtained after annealing. The annealed product was ligated with pET21a(+) / HBcAg digested by BamH I and Sac I to construct a recombinant prokaryotic expression plasmid containing the Ct MOMP epitope gene and the tandem epitope gene in the HBcAg MIR region; at the same time, the Ct MOMP Immunodominant epitopes were respectively connected to the N and C ends of the modified HBcAg, compared for research, and identified by sequencing ( figure 2 ). The results showed that: HBcAg(MIR) / Ct MOMP epitope 1, HBcAg(MIR) / Ct MOMP epitope 1+2, HBcAg(MIR) / CtMOMP epitope 1+2+3, Ct MOMP epitope 1 / HBcAg and HBc...
Embodiment 3
[0111] Example 3. Immunogenicity and immune protection effect of HBcAg / CtMOMP epitope vaccine
[0112]Female BALB / c mice aged 6-8 weeks (purchased from Shanghai Slack Experimental Animal Co., Ltd.) were randomly divided into 8 groups, 21 mice in each group, respectively: PBS control group, HBcAg group, HBcAg(MIR) / MOMP epitope 1 (MIR region carries dominant epitope 1), HBcAg(MIR) / MOMP epitope 1+2, HBcAg(MIR) / MOMP epitope 1+2+3(, Ct MOMP epitope 1 / HBcAg( N-terminal), HBcAg / Ct MOMP epitope 1 (C-terminal) and Ct MOMP epitope synthetic peptide group. Take 100 μl test samples (1tg / μl) at 0, 2, and 4 weeks respectively, and inject them into the back of the mouse for immunization Collect tail vein blood and vaginal secretions at 0w, 2w, 4w, 6w, 8w, 10w, 12w, 14w, 18w, and 20w after immunization, and detect Ct-specific IgG antibodies in serum and IgA antibodies in secretions by ELISA; In the 7th week, the spleen was taken to prepare splenocyte suspension, and the specific CTL killi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com