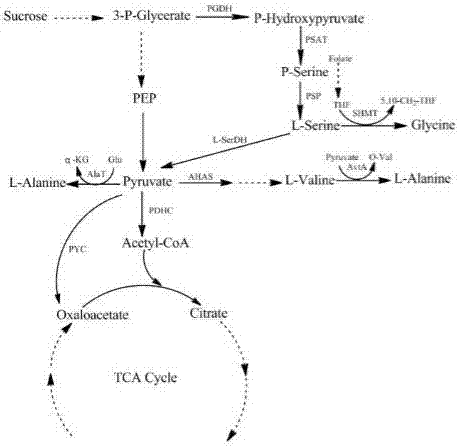
Construction of a high-yielding l-serine recombinant Corynebacterium glutamicum and its fermentation method
A technology of Corynebacterium glutamicum and serine, applied in the biological field, can solve the problems of slow growth of bacteria, decreased production efficiency, decreased production rate, etc., and achieves improved yield and sugar-acid conversion rate, increased growth rate, and increased production intensity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: Construction of recombinant bacteria Corynebacterium glutamicum 33a ΔSS ΔavtA
[0024] Based on the genome sequence information of Corynebacterium glutamicum ATCC13032, primers for constructing a recombinant knockout plasmid for avtA knockout were designed. The primer sequences are as follows:
[0025] avtA-1:5'-GAAAAGCTTAAGGCCCTGCAGTAGTG-3'(HindIII)
[0026] avtA-2:5'-CCCATCTGTTAAACTTAAACCAAACGGCTGAACATTGCTT-3'
[0027] avtA-3:5'-GGTTTAAGTTTAACAGATGGGGGTGTGCGCAAAATCGG-3'
[0028] avtA-4:5'-ATTCTAGACCTGCGCTGCCACGTTGT-3'(XbaI)
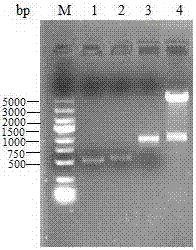
[0029] Use primers avtA-1 and avtA-2 to amplify the upstream fragments respectively, use primers avtA-3 and avtA-4 to amplify the downstream fragments, respectively purify the upstream and downstream fragments by agarose gel electrophoresis, see figure 2 , the lengths are 507bp and 548bp respectively; the upstream and downstream fragments are used as templates respectively, and the upstream and downstream fusion fragments are ampl...
Embodiment 2
[0030] Example 2: Construction of recombinant bacteria Corynebacterium glutamicum 33a ΔSS ΔavtAΔC-T ilvN
[0031] Using the recombinant strain constructed in Example 1 as the starting strain, the 249bp at the C-terminal of the coding regulatory gene ilvN of the acetohydroxyacid synthase (AHAS) that catalyzes the generation of α-acetolactate from pyruvate is precisely deleted to make acetohydroxyacid Synthase activity was reduced. The following primers were designed and synthesized for the construction of recombinant plasmids for knocking out ilvN:
[0032] ilvN-1:5'-CCCAAGCTTGCTGTTTCCAGATGACCAACC-3'(HindIII)
[0033] ilvN-2:5'-GGCGATAGTGGTCTCTTCATCAAGTCGCACGACTTTGAGC-3'
[0034] ilvN-3:5'-GAAGAGACCACTATCGCCACAGCAATTAATCTGATTGC-3'
[0035] ilvN-4:5'-CGCGGATCCCGTTCAGGTTTGGCTCGATG-3'(BamHI)
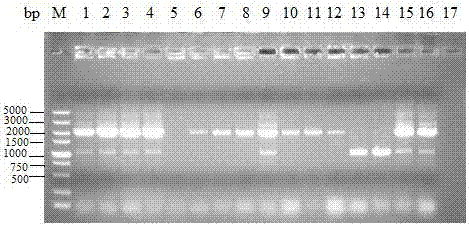
[0036] For specific experimental steps, refer to Example 1. For the construction diagram of the knockout plasmid pK18mobsacBΔC-T ilvN, see Figure 4 . The verification PCR primers of r...
Embodiment 3
[0040] Embodiment 3: the enzyme activity assay method of acetohydroxyacid synthase (AHAS)
[0041] Reaction system: 100mM potassium phosphate buffer (pH 7.3), 50mM sodium pyruvate, 10mM MgCl 2 and 100 μM flavin adenine dinucleotide (FAD). Take the Corynebacterium glutamicum cells in the logarithmic phase, centrifuge to remove the supernatant, wash 3 times with 0.2% ice KCl, ultrasonically break and centrifuge to obtain the supernatant, take a certain amount of supernatant and add it to the reaction system, Add 100 μL of 50% H after incubation at 37°C for 20 min 2 SO 4 Stop the reaction. After incubating at 37° C. for 30 minutes, acetoin was produced from α-acetolactic acid, and the content of acetoin in the reaction liquid was determined by gas chromatography. One unit of enzyme activity is set as: 1 nmol of α-acetolactate produced per milligram of protein per minute. Protein concentration was determined by the Bradford method. Acetohydroxyacid synthase enzyme activity a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com