Spinal implantation unit and preparation method thereof
A spine and medicine technology, applied in the field of spine implantation unit and its preparation, can solve the problems of drug action discount and the like, and achieve the effects of simple manufacturing method, stable chemical properties, good penetration and permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
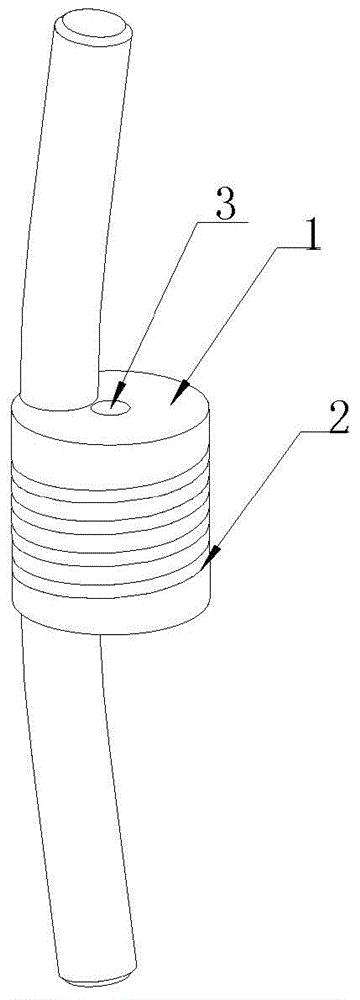
[0046] After the dynamic rod is machined, use an ultrasonic cleaning machine to clean the oil, and then use an ultrasonic cleaning machine for final cleaning and drying in a 100,000-class purification workshop. dynamic stick figure 1 shown.
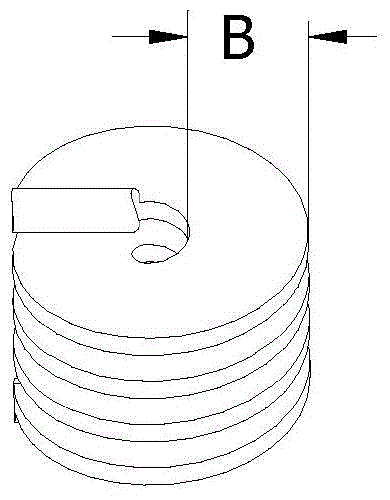
[0047] Select the liquid silicone rubber that can be implanted for a long time, first add the drug (clonidine) into the liquid silicone rubber and mix well, then add the curing agent (0.5wt%) matched with the liquid silicone rubber, and mix well to form a drug-containing The liquid silicone rubber with curing agent is transferred into the syringe and then injected into the spiral groove of the dynamic rod. Curing at room temperature for 24 hours. The shape of the cured silicone rubber body loaded with drugs is as follows: figure 2 Shown, where the thickness B is 3mm.
[0048] Liquid silicone rubber: drug: curing agent = 100:30:0.5 (weight ratio).
[0049] The daily drug release rate is controlled at less than 100 μg / kg (patient body...
Embodiment 2
[0051] Same as Example 1, except that α-adrenaline is selected as the drug and the thickness B is 2 mm. The dynamic rod is implanted near the intervertebral disc to share the activity and stress function of the intervertebral disc and prevent further degeneration of the intervertebral disc. Dynamic sticks carry alpha-adrenaline, which can treat degenerative disc disease including herniated discs.
Embodiment 3
[0053] Same as Example 1, except that liquid silicone rubber containing medicine and curing agent is injected into the through hole in the middle of the dynamic rod, the weight ratio of medicine to liquid silicone rubber is 40%, and the thickness is 5mm. Liquid silicone rubber containing drug and curing agent is injected into the through hole, which can increase the drug loading and release the drug for as long as one year.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com