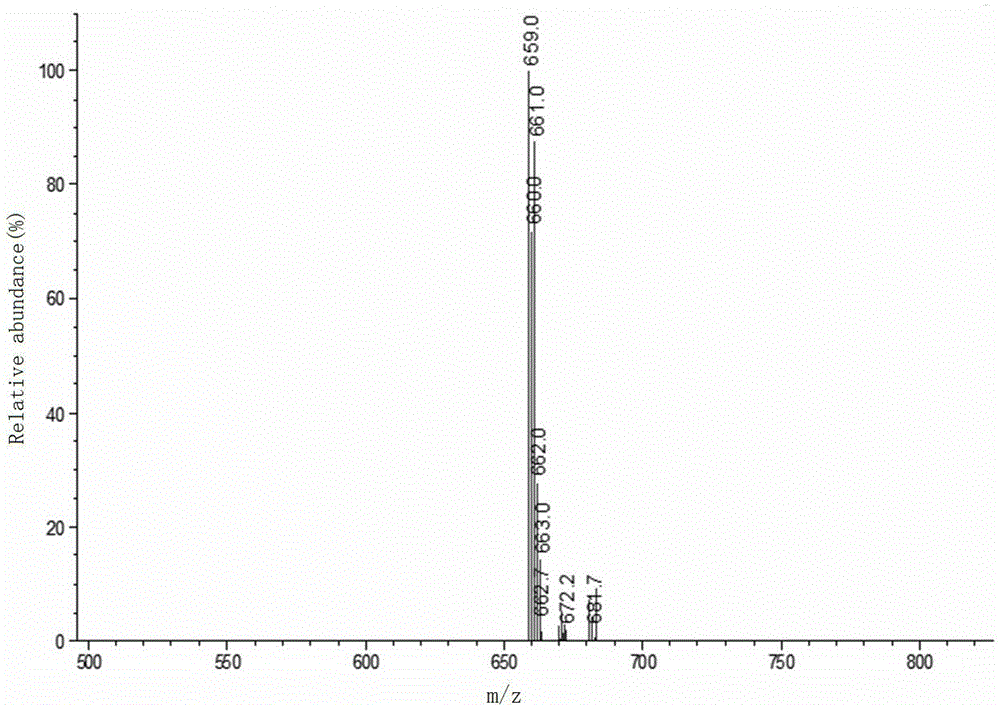
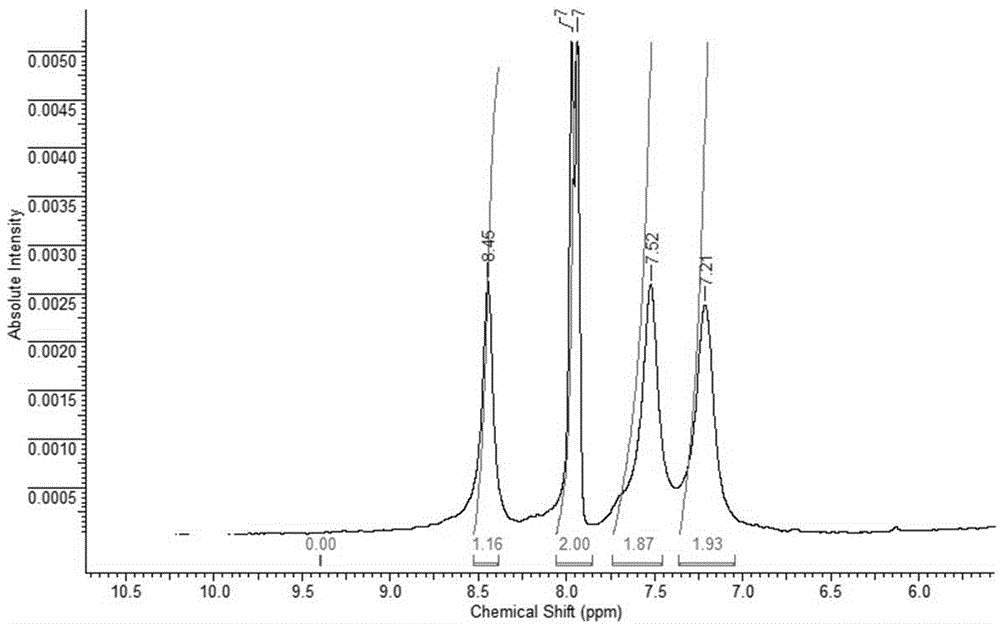
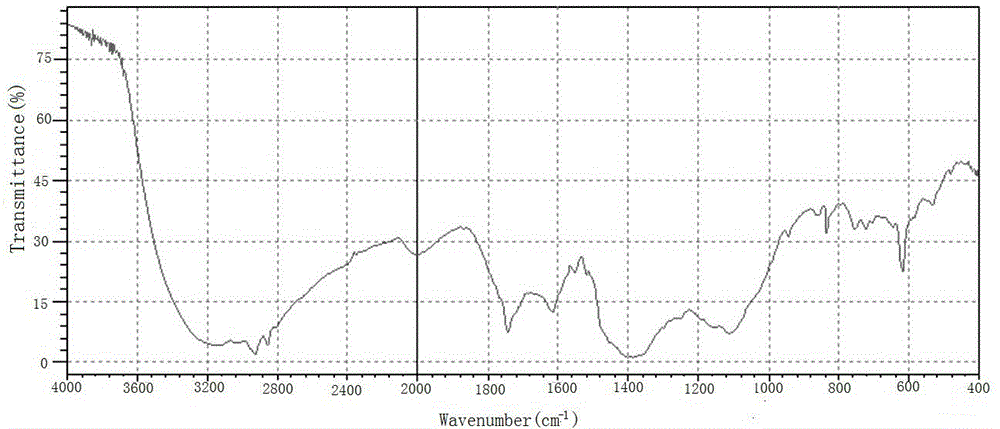
Perpetration method for novel phenophosphazineanthracene dioxazine dye and application of novel phenophosphazineanthracene dioxazine dye to wool salt-free dyeing
A technology of triphenyldioxazine and benzophosphine, which is applied in the field of outstanding performance of wool dyeing without salt, can solve the problems of the late start of research on triphenyldioxazine compounds, and achieve obvious anti-ultraviolet performance, excellent water-solubility, and excellent heat resistant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] A novel benzophosphazinatriphenyldioxazine dye, whose general structural formula is
[0057] (I)
[0058] In formula (I), R = H, HSO 3 , R 1 = H, CH 3 、C 2 h 5 , R 2 = H, CH 3 、C 2 h 5 , R 3 = H, CH 3 、C 2 h 5 , R 5 = Cl, Br, H, R 7 = Cl, Br, H.
[0059] The preparation method of novel benzophosphazinatriphenyldioxazine dye is prepared by the following steps:
[0060] Step 1, with 5,10-dihydro-phosphazine-10-oxide and its derivatives as reactants, with nitric acid and acetic acid mixed volume ratio of 1:(1-5) as nitroxy reagent, nitroxyl The molar ratio of reagents to 5,10-dihydro-phosphazine-10-oxide and its derivatives is between (1.5-4):1, the reaction temperature is between 4-15°C, after 1- The 4-hour nitration reaction gives 5,10-dihydro-phosphazine-10-oxide and its derivatives mononitrates, and the specific reaction equation is shown in formula II:
[0061] (II)
[0062] Step 2, reducing the mononitrated compound of 5,10-dihydro-phosphazine-...
Embodiment 2
[0080] A novel benzophosphazinatriphenyldioxazine dye, whose general structural formula is
[0081] (I)
[0082] In formula (I), R = H, HSO 3 , R 1 = H, CH 3 、C 2 h 5 , R 2 = H, CH 3 、C 2 h 5 , R 3 = H, CH3 、C 2 h 5, R 5 , R 7 = Cl, Br, H.
[0083] The preparation method of novel benzophosphazinatriphenyldioxazine dye comprises the steps:
[0084] Step 1 uses 5,10-dihydro-phosphazine-10-oxides and derivatives thereof as reactants, with nitric acid and acetic acid mixed in a volume ratio of 1: 2 as nitroxyl reagent mixture as nitroxyl reagent, nitroxyl The molar ratio of the reagents to 5,10-dihydro-phosphazine-10-oxide and its derivatives is 3:1, the reaction temperature is 15°C, and the reaction time is 4 hours to obtain 5,10-di Hydrogen-phosphazine-10-oxides and the mononitrides of their derivatives, the specific reaction equation is shown in formula II:
[0085] (Ⅱ)
[0086] In formula (Ⅱ), R 1 = H, R 2 = H, R 3 = H;
[0087] In step 2, the mononit...
Embodiment 3
[0108] A novel benzophosphazinatriphenyldioxazine dye, whose general structural formula is
[0109] (I)
[0110] In formula (I), R = H, HSO 3 , R 1 = H, CH 3 、C 2 h 5 , R 2 = H, CH 3 、C 2 h 5 , R 3 = H, CH 3 、C 2 h 5, R 5 , R 7 = Cl, Br, H.
[0111] The preparation method of novel benzophosphazinatriphenyldioxazine dye comprises the steps:
[0112] Step 1 uses 5,10-dihydro-phosphazine-10-oxide and its derivatives as reactants, nitric acid and acetic acid at a mixing ratio of 1:5 as nitroxyl reagent, nitroxyl reagent and 5,10- The molar ratio of dihydro-phosphazine-10-oxide and its derivatives is 1.5:1, the reaction temperature is 10°C, and the reaction time is 2 hours to obtain 5,10-dihydro-phosphazine -10-Oxide and the mononitration of its derivatives, the specific reaction equation is shown in formula II:
[0113] (Ⅱ)
[0114] In formula (Ⅱ), R 1 = H, R 2 =H 5 , R 3 = H;
[0115] In step 2, the mononitrated compound of 5,10-dihydro-phosphazine-10-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com