Anti-inflammatory application and extraction method of 6-hydroxyl-eremophilane-7(11)-en-12,8-lactone in ligularia sibirica
A technology of cucurbit seven and hydroxyl group, applied in the field of natural product chemistry, can solve the problems of low extraction efficiency, no report on anti-inflammatory activity research, etc., and achieve a significant effect of anti-inflammatory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Get the dried root and rhizome coarse powder 4kg of Gourd Seven, use the volume fraction as 95% ethanol cold soaking to extract three times, the volume (L) that adds three times is respectively the root of Gourd Seven and the rhizome coarse powder mass (kg) of 6,4 and 4 times, cold soaking for three days each time, stirring three times a day, 10 minutes each time. Filtrate, discard the medicinal residues, combine the extracts, recover the solvent, and obtain 433.0 g of brown ethanol extract.
[0037] After mixing the obtained ethanol extract with diatomaceous earth, first degrease with petroleum ether (60-90 ℃) Soxhlet extraction, remove the strong lipophilic part, then carry out Soxhlet extraction with dichloromethane as solvent for 18 hours, and recover Solvent, concentrated to dichloromethane extract, a total of 160.6g.
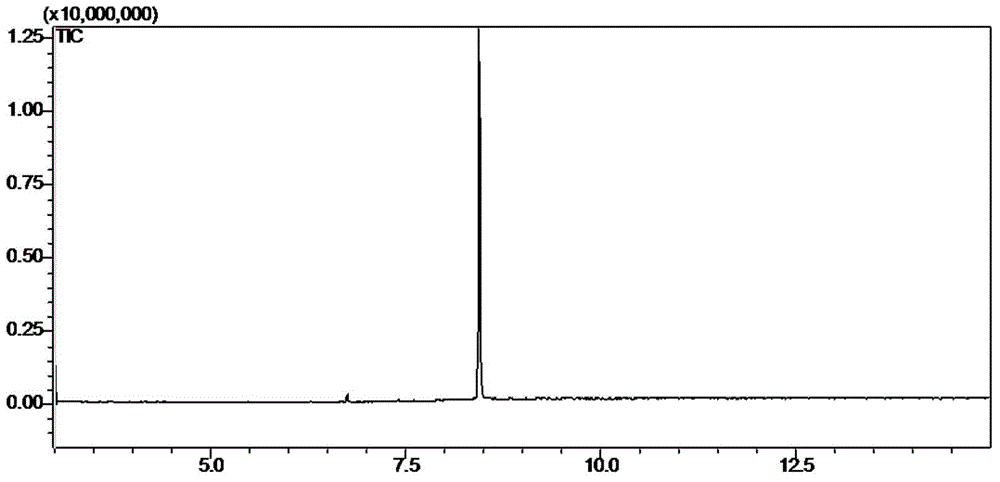
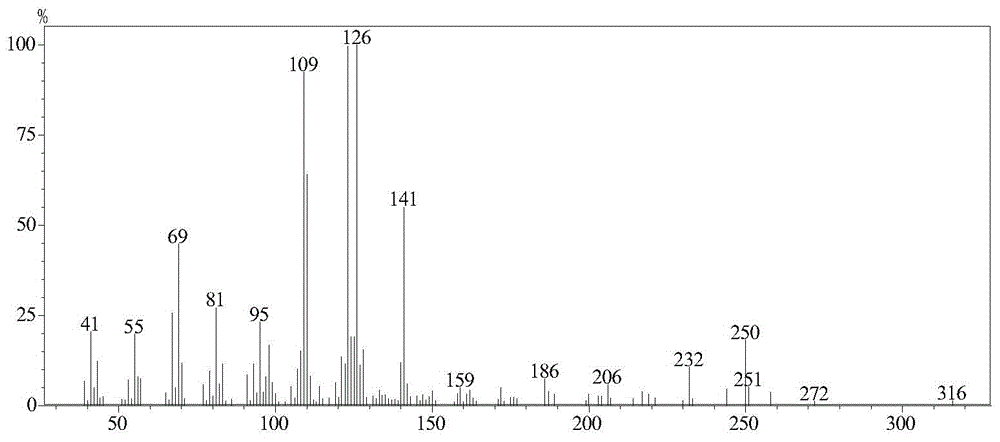
[0038] Take 55.0 g of dichloromethane extract, and perform column chromatography on a silica gel column, using petroleum ether (60-90°C)-ethyl aceta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com