Aspirin-containing anti-brain stroke solid oral preparation
An oral preparation and aspirin technology, applied in the field of pharmaceutical preparations, can solve the problems of no aspirin stroke, no chlorzoxazone anti-stroke, etc., and achieve the effects of improving neurological deficits and preventing the expansion of infarct size.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
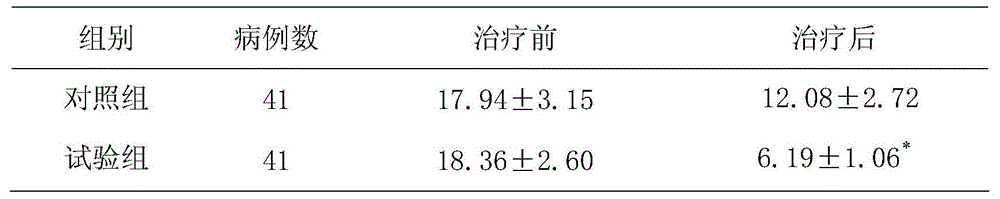
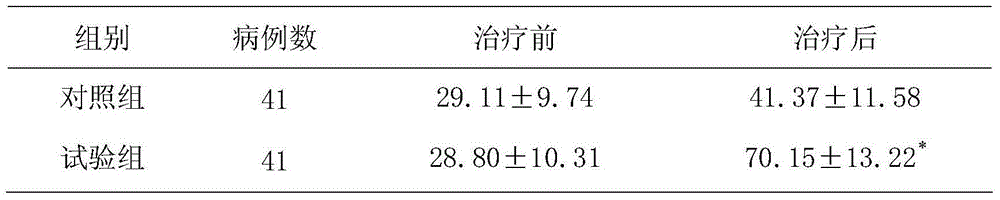
[0016] Example 1: Clinical efficacy of aspirin combined with chlorzoxazone on progressive stroke
[0017] 82 cases of patients with progressive stroke. Inclusion criteria: ①Meet the diagnostic criteria adopted by the National Cerebrovascular Disease Academic Conference in 1995, and confirmed by cranial MRI as new cerebral infarction, excluding cerebral hemorrhage; ②Focal neurological function within 48 hours of onset Progressive aggravation of defect symptoms; ③No serious systemic complications; ④Those with bleeding history, peptic ulcer or blood system disease and those with bleeding tendencies are not eligible; ⑤Those with impaired consciousness are not eligible; Cannot be selected. According to the method of random grouping, all patients were randomly divided into the test group and the control group, with 41 cases in each group. The test group had 25 males and 16 females, with an average age of 69.5±8.2 years; the control group had 41 cases, 24 males, There were 17 female...
Embodiment 2
[0026] Example 2: Effect test of chlorzoxazone on simple right middle cerebral artery occlusion model
[0027] 40 SPF grade SD rats, male, body weight 200-240g. Rats were randomly divided into normal control group, model control group, low-dose and high-dose chlorzoxazone groups, with 8 rats in each group. After the test started, the low-dose and high-dose groups of chlorzoxazone were given 60 and 180 mg / kg of chlorzoxazone by intragastric administration respectively, and the normal control group and the model control group were given 10 mL / kg of normal saline by intragastric administration. Each group was administered once a day for 7 consecutive days. 3 hours after the last administration, except for the normal control group, the rats in other groups were established as follows in the simple right middle cerebral artery occlusion model (MCAO model): chloral hydrate 400mg / kg intraperitoneal injection of anesthetized rats, and then supine position Fix it on the operating tab...
Embodiment 3
[0028] Example 3: Preparation of compound aspirin enteric-coated tablets
[0029]
[0030] Preparation process: pass aspirin and chlorzoxazone through a 80-mesh sieve, and other excipients through a 100-mesh sieve, mix aspirin, chlorzoxazone, and mannitol according to the prescription ratio, add an appropriate amount of distilled water to granulate, and add carboxymethyl after drying Sodium starch glycolate and magnesium stearate are blended into granules, pressed into 1000 tablets, and then coated with enteric coating.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com