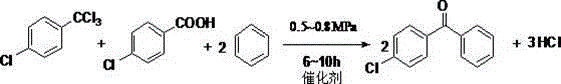Method for preparing p-chlorodiphenyl ketone
A technology of p-chlorobenzophenone and p-chlorobenzoic acid is applied in the field of preparation of p-chlorobenzophenone, can solve the problems of large amount of catalyst, many three wastes and high yield, and achieves reduction of reaction steps and equipment investment. , to avoid the effect of multi-step reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0020] 6 moles of benzene, 1.5 moles of p-chlorobenzoic acid, 1.5 moles of p-chlorobenzotrichloride and 0.006 moles of ferric chloride were mixed in a 1-liter pressure reactor. The lid of the reaction kettle was tightened and placed in a hot bath with an electromagnetic stirring speed of 50-150rpm. All valves were closed at the beginning of the reaction, hydrogen chloride gas was released from the gas escape valve and in turn received into the ice bath cooled sodium hydroxide absorbent. Start heating for about 30 minutes and the temperature reaches 160°C. As the temperature increases, the pressure on the reactor increases to 0.5MPa. Open the gas escape valve on the top of the reactor to let the hydrogen chloride gas slowly escape into the sodium hydroxide solution. The pressure is maintained React at 0.75 MPa, the temperature is 175 ° C, after 8 hours of reaction, basically no hydrogen chloride gas escapes, the reaction substance is cooled to room temperature, then the reactio...
example 2
[0022] 5 moles of benzene, 1 mole of p-chlorobenzoic acid, 1 mole of p-chlorotrichlorotoluene and 0.004 moles of zinc chloride were mixed in a 1-liter pressure reactor. The reaction pressure was maintained at 0.7 MPa, the temperature was 165° C., and the reaction was carried out for 6 hours. Other operations were the same as in Example 1. The yield of p-chlorobenzophenone is 89%.
example 3
[0024] 6 moles of benzene, 1.5 moles of p-chlorobenzoic acid, 1.5 moles of p-chlorotrichlorotoluene and 0.005 moles of zinc benzoate were mixed in a 1-liter pressure reactor. Pressure 0.5MPa, temperature 150 ℃, reacted 10 hours, other operations were with example 1, and the yield of p-chlorobenzophenone was 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com