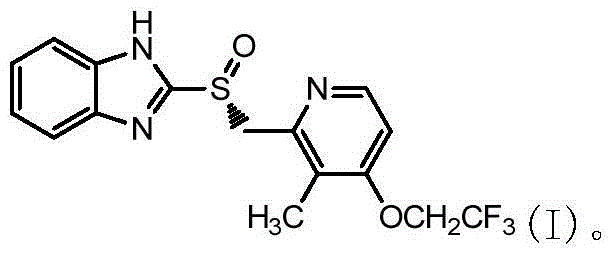
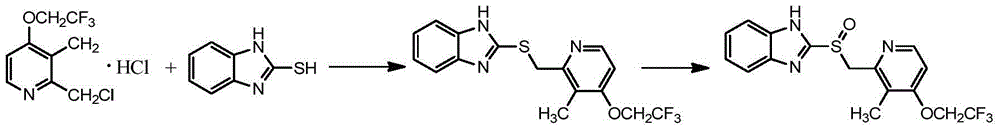
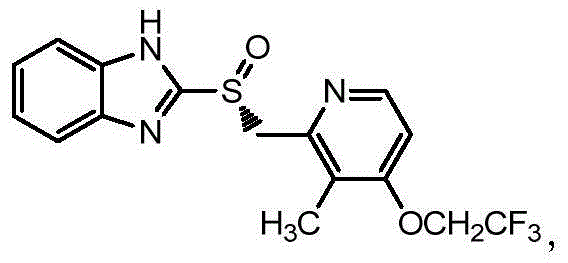
Asymmetric oxidation method for dexlansoprazole
An asymmetric and oxidant technology, applied in the direction of asymmetric synthesis, organic chemical methods, chemical instruments and methods, etc., can solve the problems of cumbersome operation and unrecoverable solvents, so as to avoid excessive oxidation, simplify the post-reaction treatment process, and reduce costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Post-reaction treatment of 2-[[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylsulfinyl]-1H-benzimidazole
[0031] At 20°C, 2-[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridine]methylsulfanyl-1H-benzimidazole (30g) / 300ml toluene (Industrial grade) into a dry and clean 1000ml four-necked round bottom flask. Add 25.3g of L-(+)-diethyl tartrate / 16.3g of tetraisopropyl titanate, 7.4g of N,N-diisopropylethylamine, and 21.0g of cumene hydroperoxide to react, and take samples to monitor the reaction. After completion of the reaction, add 96g of 30% Na in the system 2 S 2 o 3 The solution quenched the reaction. Separate the layers to obtain the toluene phase. At 15°C, extract with 10% diethylamine solution (285ml×3), combine the aqueous phase, add 22.5g of acetone to the aqueous phase, and stir for 15min. At 15°C, use 20% acetic acid solution to adjust the pH to about 7, and continue stirring for 2 h. Suction filtration and drying to obtain 2-[[3-methyl-4-(2,...
Embodiment 2
[0032] Example 2 Post-reaction treatment of 2-[[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylsulfinyl]-1H-benzimidazole
[0033] At 20°C, 2-[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridine]methylsulfanyl-1H-benzimidazole (30g) / 300ml toluene (Industrial grade) into a dry and clean 1000ml four-necked round bottom flask. Add 25.3g of L-(+)-diethyl tartrate / 16.3g of tetraisopropyl titanate, 7.4g of N,N-diisopropylethylamine, and 21.0g of cumene hydroperoxide to react, and take samples to monitor the reaction. After completion of the reaction, add 96g of 30% Na in the system 2 S 2 o 3 The solution quenched the reaction. Separate the layers to obtain the toluene phase. At 15°C, 30% diethylamine solution was used for extraction (285ml×3). Combine the water phases, add 22.5 g of acetone to the water phase, and stir for 15 min. At 15°C, adjust the pH to about 7 with 20% acetic acid solution. The reaction continued to stir for 2h. Suction filtration and drying to obtain ...
Embodiment 3
[0034] Example 3 Post-reaction treatment of 2-[[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylsulfinyl]-1H-benzimidazole
[0035]At 20°C, 2-[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridine]methylsulfanyl-1H-benzimidazole (30g) / 300ml toluene (Industrial grade) into a dry and clean 1000ml four-necked round bottom flask. Add 25.3g of L-(+)-diethyl tartrate / 16.3g of tetraisopropyl titanate, 7.4g of N,N-diisopropylethylamine, and 21.0g of cumene hydroperoxide to react, and take samples to monitor the reaction. After completion of the reaction, add 245g 20% Na in the system 2 S 2 o 3 The solution quenched the reaction, and the layers were separated. The obtained toluene phase was separated and extracted with 8% diethylamine solution (470ml×3). Take the combined aqueous phase and measure it by HPLC. Add 37.5 g of acetone to the water phase, and after cooling the system to 15° C., add 20% acetic acid solution to adjust the pH to about 8, and continue stirring for 1 h. S...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com