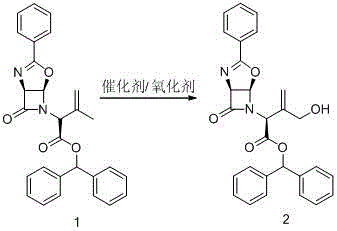
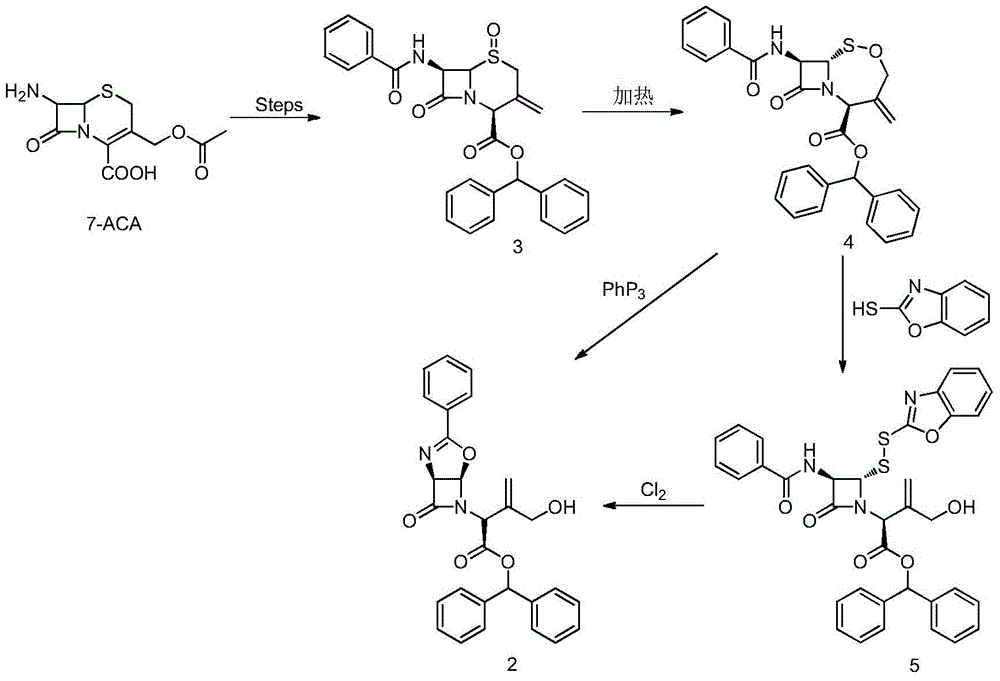
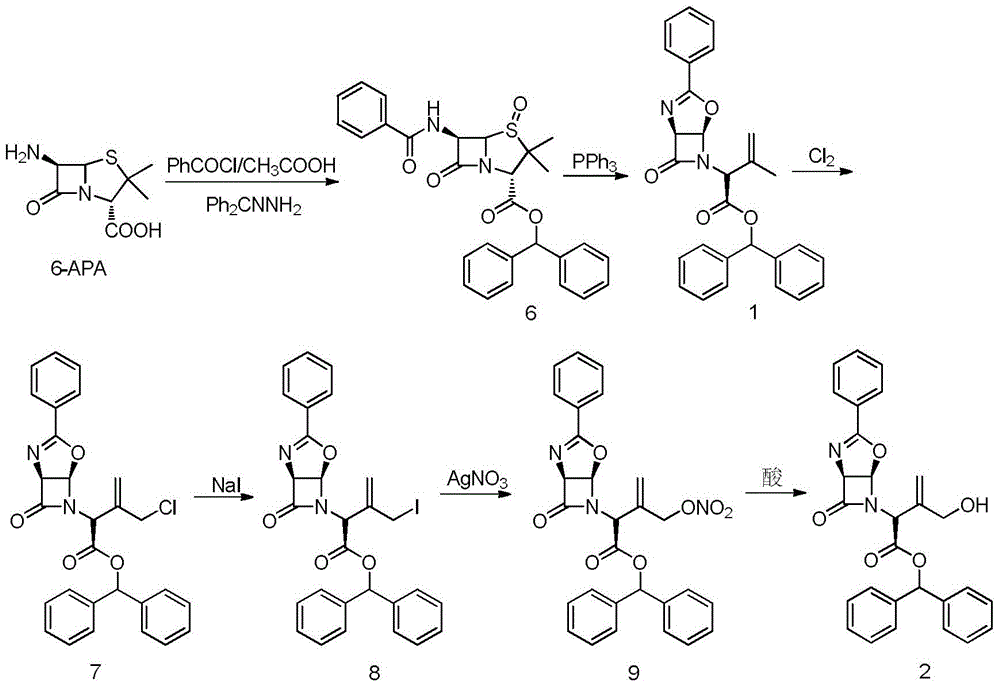
A kind of allyl position hydroxylation prepares the method for the key intermediate of oxycephem antibiotics
A technology of oxycephem and allyl, which is applied in the direction of organic chemistry, can solve the problems of unfavorable post-treatment removal, many side reactions, and poor repeatability of the pilot test, so as to avoid the use of chlorine-resistant equipment and light-proof equipment, Improvement of production competitiveness and reduction of production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 10g of intermediate 1 was dissolved in 80ml of dichloromethane, 3g of selenium dioxide and 0.4g of tetrabutylammonium bromide were added, and reacted at room temperature for 2h. Cool to -5°C, slowly add 7ml of tert-butanol peroxide, and TLC detects that the reaction is complete. At room temperature, the system was poured into 100ml of 20% sodium sulfite aqueous solution, stirred for 1 hour, and allowed to stand to separate layers. The organic phase was washed once with 80 ml of saturated sodium bicarbonate solution, 80 ml of water, and 80 ml of saturated brine, and finally dried by adding 7 g of anhydrous sodium sulfate for 10 h. After suction filtration, the filtrate was evaporated to dryness under reduced pressure, and the residue was recrystallized with 5 ml of ethanol to obtain Intermediate 2. Yield 33%.
[0035] The NMR data of intermediate 1 are as follows: 1 HNMR (400MHz, CDCl 3 )δ7.92(d, J=7.3Hz, 2H), 7.55~7.30(m, 13H), 6.93(s, 1H), 6.34(d, J=3.3Hz, 1H), 5.3...
Embodiment 2
[0038] 100g of intermediate 1 was dissolved in 800ml of dichloromethane, 30g of selenium dioxide and 4g of tetrabutylammonium bromide were added, and reacted at room temperature for 2h. Cool to -5°C, slowly add 70ml of tert-butanol peroxide, and TLC detects that the reaction is complete. At room temperature, the system was poured into 1 L of 20% sodium sulfite aqueous solution, stirred for 1 h, and allowed to stand to separate layers. The organic phase was washed once with 800 ml of saturated sodium bicarbonate solution, 800 ml of water, and 800 ml of saturated brine, and finally dried by adding 70 g of anhydrous sodium sulfate for 10 h. After suction filtration, the filtrate was evaporated to dryness under reduced pressure, and the residue was recrystallized with 60ml of ethanol to obtain Intermediate 2. Yield 32%.
Embodiment 3
[0040] 1kg of intermediate 1 was dissolved in 10L of dichloromethane, 300g of selenium dioxide and 40g of tetrabutylammonium bromide were added, and reacted at room temperature for 2h. Cool to -5°C, slowly add 700ml tert-butanol peroxide, and TLC detects that the reaction is complete. At room temperature, the system was poured into 8L of 20% sodium sulfite aqueous solution, stirred for 1 h, and allowed to stand to separate layers. The organic phase was washed once with 8L of saturated sodium bicarbonate solution, 8L of water, and 8L of saturated brine, and finally dried by adding 700g of anhydrous sodium sulfate for 10 hours. After suction filtration, the filtrate was evaporated to dryness under reduced pressure, and the residue was recrystallized with 600ml of ethanol to obtain Intermediate 2. Yield 30%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com