Application of natural small molecular compound in inflammation and tumor resistance
A small molecule compound, small molecule technology, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Implementation 1: Cell Culture Technology
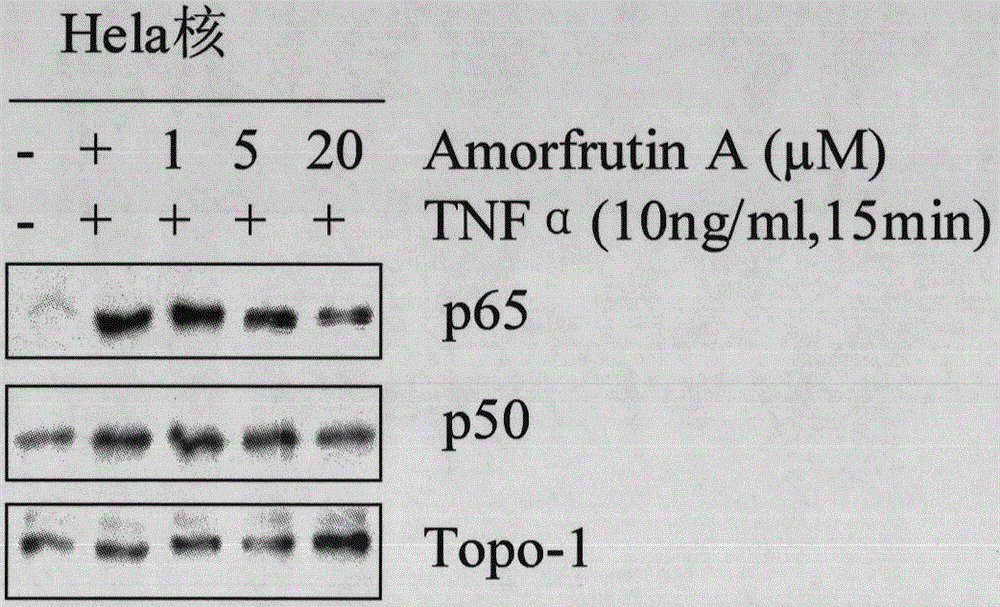
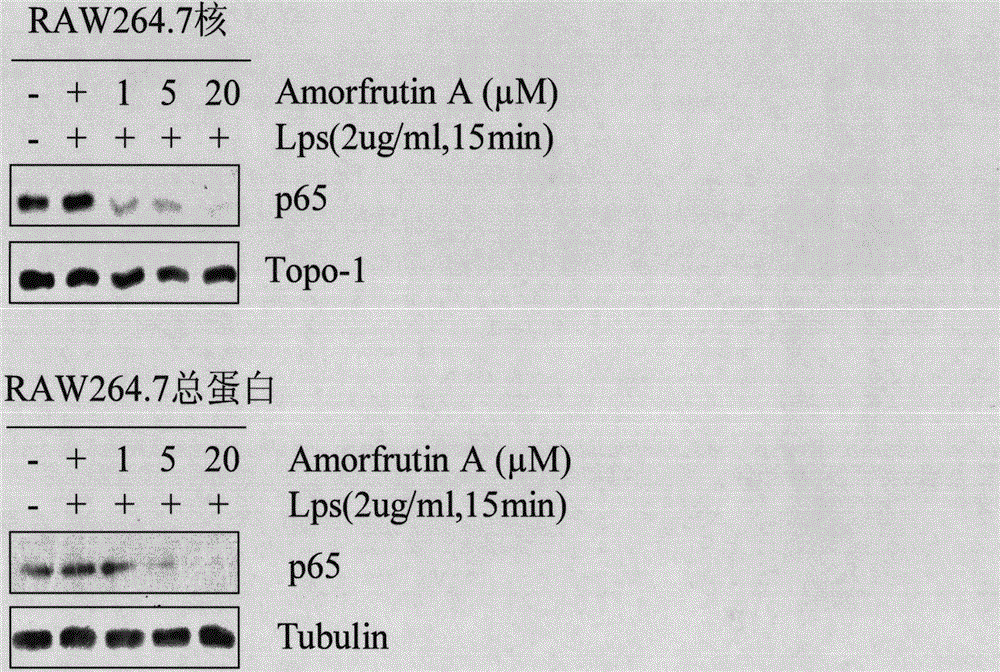
[0029] After the cells entered the logarithmic growth phase, they were digested with 0.25% trypsin, and the number of cells was 5 × 10. 4 The density was evenly divided into 5 cell culture dishes with a diameter of 6 cm, and 3 ml of DMEM medium (containing inactivated 10% calf serum, 100 U / L penicillin) was added to each culture dish and placed in a constant temperature incubator (37 ° C, 5% CO 2, normal oxygen concentration and saturated humidity). After the cells were completely attached to the wall, the cells were divided into negative control group, positive control group, and concentration gradient group (1 μM, 5 μM, 20 μM). After culturing for 4 hours, the positive control group and concentration gradient group were treated with TNFα 3 μl (final concentration 10 ng / ml, Hela cells were treated with TNFα, RAW264.7 cells were treated with LPS), and cultured for 15 minutes.
Embodiment 2
[0030] Implementation 2: Westernblot method
[0031] (1) Preparation of protein samples, including extraction of total protein and nuclear protein.
[0032] (2) Protein denaturation: 15 μl of sample protein was taken and added to corresponding tubes, 5 μl of loading buffer was added, and boiled for 5 minutes.
[0033] (3) Place the boiled protein sample on an SDS-PAGE gel for electrophoresis.
[0034] (4) After the electrophoresis stops, the protein sample on the gel is transcribed onto a methanol-soaked PVDF membrane.
[0035] (5) Put the PVDF membrane into 5% skimmed milk and seal it at room temperature for 1 hour.
[0036] (6) Primary antibody hybridization, secondary antibody hybridization.
[0037] (7) ECL luminescent kit detection.
Embodiment 3
[0038] Implementation 3: PCR method
[0039] (1) Total RNA extraction:
[0040] The drug-treated cells were repeatedly washed with PBS three times, 1ml of Ttiol was added, and the cells were blown repeatedly with a syringe to lyse them all. Add 200 μl CCl 4 The solution was separated into layers, and centrifuged at 12000 r for 15 min at 4°C. Take the upper layer into a new tube, add 500 μl of isopropanol, mix well, and centrifuge at 12000r at 4°C for 10 minutes. Carefully discard the supernatant, add 1ml of 75% ethanol to the tube containing RNA, shake, and centrifuge at 12000r at 4°C for 5min. Fully discard the supernatant, and place the ultra-clean bench to ventilate and dry. After being thoroughly dried, add 30 μl DEPC-DDW to dissolve it completely.
[0041] (2) Using RNA as a template to synthesize cDNA by reverse transcription.
[0042] Table 1
[0043]
[0044] RT-PCR reaction conditions:
[0045]
[0046] (3) PCR reaction: double-stranded DNA is synthesize...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com