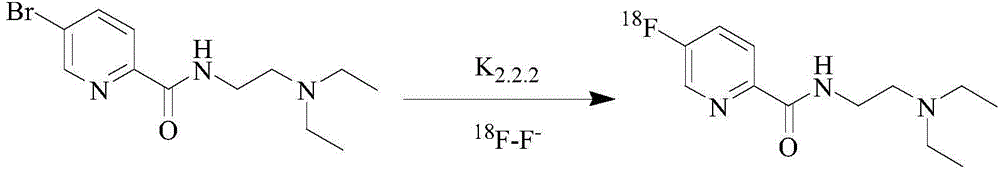Preparation process of positron imaging agent 18F-5-floro-N-(2-(diethylamino) ethyl) pyridinecarboxamide
A technology of 18F-5- and positron imaging agents, applied in the direction of radioactive carriers, etc., can solve the problems of difficulty in obtaining stable output, long process time, and large radiation dose, and achieve easy automatic operation and simple preparation process , the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] provided by the invention 18 The synthetic route of F-5-fluoro-N-(2-(diethylamino) ethyl) pyridinecarboxamide is as follows figure 1 Shown, the precursor is 5-bromo-N-(2-(diethylamino) ethyl) pyridine carboxamide, can refer to literature (Liu, H.et al., Development of[ 18 F]-labeled picolinamide probes for PET imaging of malignant melanoma. J Med Chem, 2013, 56, 895-901) synthesis.
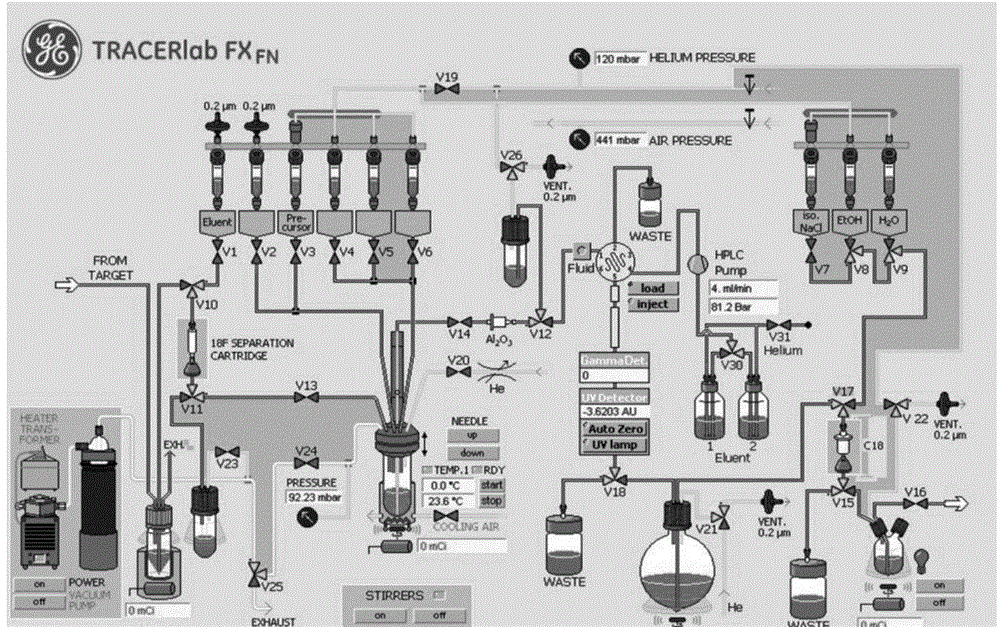
[0033] provided by the invention 18 The pipeline diagram of the preparation process of F-5-fluoro-N-(2-(diethylamino)ethyl)pyridinecarboxamide is attached figure 2 As shown, the specific process is as follows:
[0034] 1. Preparation before automatic synthesis:
[0035] 1. Short-circuit the valve V17 and valve V15 in the pipeline.
[0036] 2. Add 0.6ml of eluent to the container bottle controlled by valve V1, the eluent uses 2.8mgK 2 CO 3 , 12mg phase transfer catalyst kryptofix.k222, 300μl sterile water and 1ml acetonitrile configuration;
[0037] Add 5 mg of the precursor 5-bromo...
Embodiment 2
[0048] The present invention has investigated the preparation of the embodiment of the present invention 1 18 The cell uptake of F-5-fluoro-N-(2-(diethylamino)ethyl)pyridinecarboxamide, the specific steps are as follows: Take B16F10 and A375m cells in logarithmic growth phase and plate them, 1×10 per well 5 cells. Added to each well 18 F-5-fluoro-N-(2-(diethylamino)ethyl)pyridinecarboxamide (2μCi / well) set 3 duplicate wells in each group, incubate at 37°C for 30min, 60min and 120min respectively, absorb the radioactive medium , washed twice with PBS and collected in the same test tube. Cells were lysed with 1N NaOH and then washed twice with PBS and collected in the same test tube. Finally, the radioactive counts of the supernatant and cell lysate were measured with an automatic gamma counter. Results are expressed as cellular uptake rates:
[0049] Cell uptake rate (%)=Counts cell lysate / (Counts cell lysate+Counts supernatant)×100%.
[0050] for cell blocking studiesfor ...
Embodiment 3
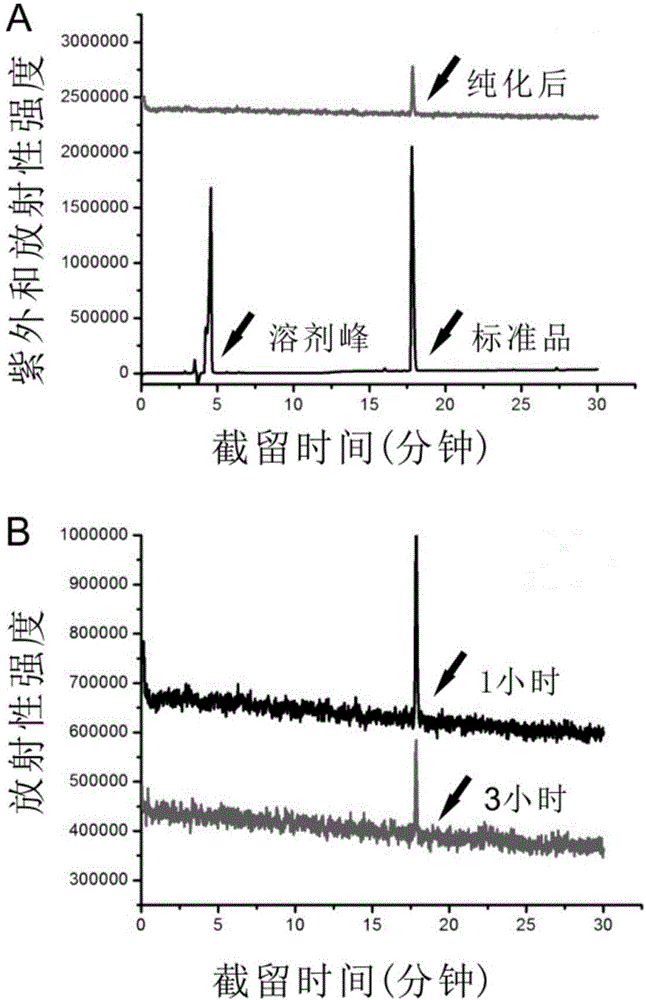
[0056] The present invention utilizes the tumor-bearing mouse model to investigate the preparation of the embodiment of the present invention 1 18 PET imaging and blocking imaging of F-5-fluoro-N-(2-(diethylamino)ethyl)pyridinecarboxamide, the specific steps are as follows:
[0057] Animal tumor model construction: C57BL / 6 mice (female, 5-6 weeks old) and BALB / C nude mice (female, 3-4 weeks old) were provided by Beijing Huafukang Biotechnology Co., Ltd. There is no special pathogen barrier system in the experimental center. All animals used in the experiment were approved by the Experimental Animal Use and Management Committee of Tongji Medical College, Huazhong University of Science and Technology. B16F10 cells 2×10 5 Suspended in 150 μl PBS, planted subcutaneously in the axilla of the right upper limb of C57BL / 6 mice, A375m cells in 2×10 6 One was suspended in 150 μl of PBS, subcutaneously planted in the armpit of the right upper limb of nude mice, and can be used for ani...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com