Chiral porous organic case quartz capillary column used for optical isomer splitting
A technology of quartz capillary and optical isomers, which is applied in the direction of solid adsorbent liquid separation, separation methods, and other chemical processes, can solve the problems of optical isomers not being separated, and achieve low cost of column manufacturing and thermal stability Good, high-resolution results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
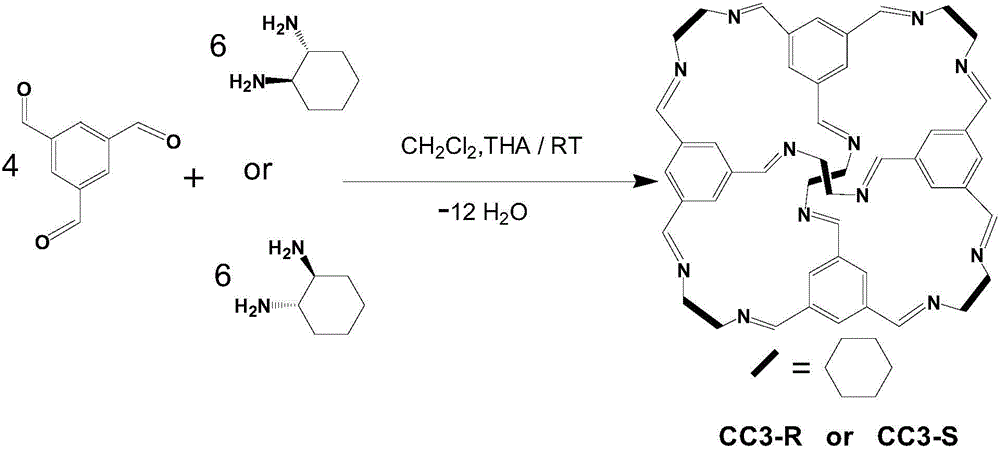
[0019] (1) Synthesis of chiral porous organic material CC3-R (such as figure 1 shown): take 1.0g of 1,3,5-trimesaldehyde, slowly add 20mL of dichloromethane and 20μL of trifluoroacetic acid without stirring, then add 20mL of 1.0g(R,R)-1, A dichloromethane solution of 2-cyclohexanediamine was slowly added thereinto, and after standing for reaction at room temperature for 3 days, octahedral white crystals grew on the inner wall of the reaction vessel. The crystals were taken out and washed 5 times with ethanol / dichloromethane (95 / 5, v / v) and then dried to obtain the chiral porous organic material CC3-R.
[0020] (2) Take the quartz capillary column and fill it with 0.1mol / L sodium hydroxide solution and keep it for 2 hours, wash it with deionized water until the eluate is neutral, then wash it with 0.1mol / L hydrochloric acid for 30 minutes, and then use Rinse with deionized water until the eluate is neutral, and pass through dry nitrogen at 120°C for 30 minutes before use;
[...
Embodiment 2
[0023] Repeat Example 1, with the following differences:
[0024] (1) Synthesis of chiral porous organic material CC3-S (such as figure 1 shown): Take 1.0g of 1,3,5-tricarbaldehyde and slowly add 20mL of dichloromethane and 20μL of trifluoroacetic acid without stirring, then add 20mL of 1.0g of (S,S)-1,2 -The dichloromethane solution of cyclohexanediamine was slowly added thereinto, and after standing for reaction at room temperature for 3 days, octahedral white crystals grew on the inner wall of the reaction vessel. The crystal was taken out and washed 5 times with ethanol / dichloromethane (95 / 5, v / v) and then dried to obtain the chiral porous organic material CC3-S.
[0025] (3) The chiral porous organic material CC3-S prepared in step (1) was prepared into a 3.0 mg / mL dichloromethane solution.
Embodiment 3
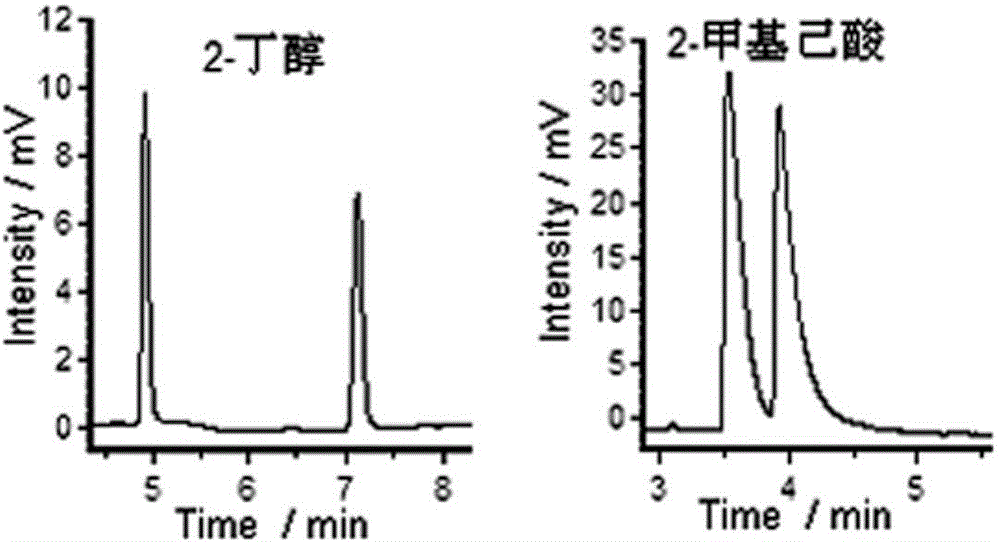
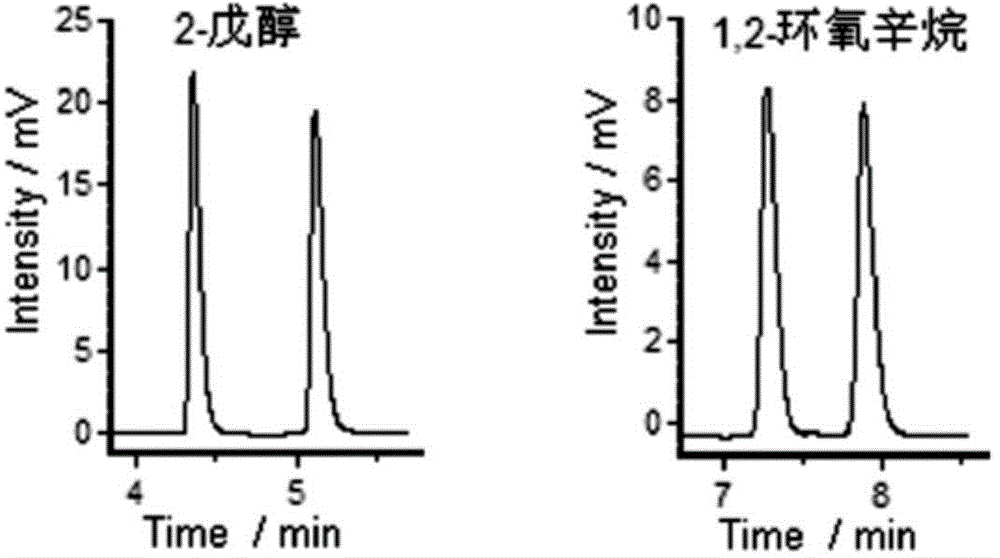
[0027] Test the resolution effect of 2-butanol and 2-methylhexanoic acid with the chiral porous organic cage quartz capillary column gained in Example 1, the chromatogram of resolution is shown in figure 2 .
[0028] Depend on figure 2 It can be seen that the chromatographic column of the present invention has better chiral resolution performance for the two optical isomers selected in the above test.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com