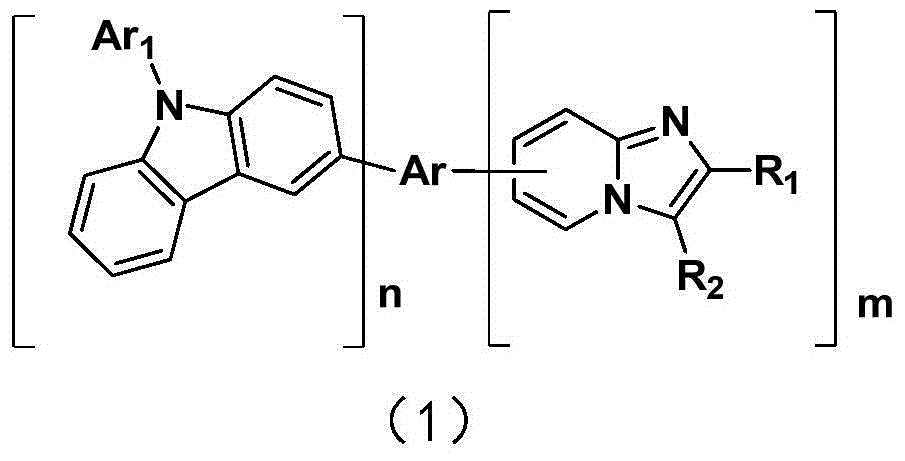
Compound containing imidazopyridine group and application thereof in organic electroluminescence
A technology of pyridine groups and electroluminescent devices, applied in luminescent materials, organic chemistry, circuits, etc., can solve the problems of shortened device life, small molecular weight, no bright spots, etc., and achieve high glass transition temperature and good thermal stability , The effect of improving the service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of compounds of the following formula
[0034]
[0035] first step,
[0036]
[0037] In a 50ml flask, add 0.892g (5mmol) of 2-amino-5-bromopyridine, 1.7g of 2-bromo-2-phenylacetophenone (6mmol), 0.491g (6mmol) of sodium bicarbonate, isopropyl 15ml of alcohol, reflux and stir for 12hrs, distill off isopropanol, add 30ml of isopropanol and 60ml of dichloromethane, collect the organic phase, separate the product by column chromatography, rinse with petroleum ether and ethyl acetate, 3:1 ratio. Obtained 1.17g, 67% yield, mp=198-1990C.
[0038] second step,
[0039]
[0040] In a 500ml three-neck flask equipped with magnetic stirring and protected by Ar gas, add 14.9g of 2,3-diphenyl-6-bromoimidazo[1,2,a]pyridine (molecular weight 348, 0.0428mol) and 120ml of THF, cool to -78°C, add 25ml of 2M nBuLi (0.05mol) dropwise, keep the temperature at -78°C, stir for 10min, then add 30ml of B(OiPr) dropwise at -78°C 3 (0.153mol), stirred to room temperature an...
Embodiment 2
[0046] Synthesis of compound 1
[0047] The synthetic process is divided into two steps, and the first step is the same as the first step in Example 1, except that 2-bromo-2-phenyl-1-(p-bromophenyl)ethanone is used to replace 2-bromo-2-phenylbenzene Ethanone is used as a raw material, and other raw materials and processes are unchanged to obtain a dibromo intermediate; the synthesis of the second step is the same as the third step of Example 1, except that 2,3-diphenylimidazo[1,2 , a] Pyridine-6-boronic acid is replaced by N-phenylcarbazole-3-boronic acid, and 3,5-bis(carbazol-9-yl)bromobenzene is replaced by 2-(p-bromophenyl)-3-benzene Base-6-bromoimidazo[1,2,a]pyridine, and other raw materials and processes remain unchanged, to obtain compound 1.
[0048] Product MS (m / e): 752, elemental analysis (C 55 h 36 N 4 ): theoretical value C: 87.74%, H: 4.82%, N: 7.44%; measured value C: 87.75%, H: 4.84%, N: 7.41%.
Embodiment 3
[0050] Synthesis of Compound 2
[0051] The synthesis process is divided into two steps, the first step is the same as the first step in Example 1, just replace 2-bromo-2-phenylacetophenone with 2-bromo-2-(p-bromophenyl)acetophenone as raw material , other raw materials and processes remain unchanged to obtain a dibromo intermediate; the synthesis of the second step is the same as the third step of Example 1, except that the 2,3-diphenylimidazo[1,2,a]pyridine -6-boronic acid for N-phenylcarbazole-3-boronic acid and 3,5-bis(carbazol-9-yl)bromobenzene for 2-phenyl-3-(p-bromophenyl)-6- Bromoimidazo[1,2,a]pyridine, the other raw materials and processes remain unchanged to obtain compound 2.
[0052] Product MS (m / e): 752, elemental analysis (C 55 h 36 N 4 ): theoretical value C: 87.74%, H: 4.82%, N: 7.44%; measured value C: 87.72%, H: 4.86%, N: 7.42%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com