Novel method for preparing crylic acid modified resin for two-component waterborne polyurethane adhesive
A polyurethane adhesive, water-based two-component technology, applied in polyurea/polyurethane adhesives, adhesives, adhesive types, etc., can solve problems such as reduced bonding performance, fluctuations in bonding performance, and damage to the ecological environment , to achieve the effects of suppressing the occurrence of defects, reducing construction difficulty and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
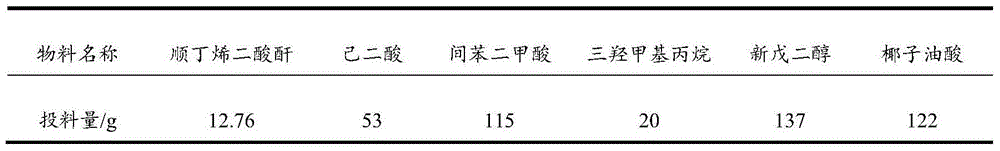
[0026] Table 1 Polyacid and polyalcohol feed list for partial synthesis of polyester
[0027]
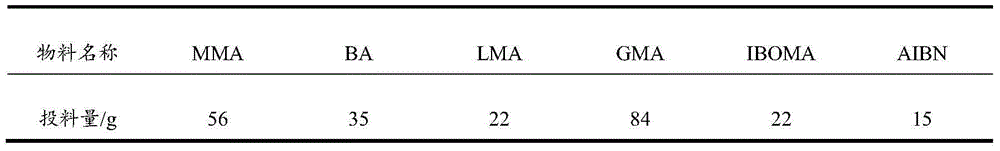
[0028] Table 2. Acrylic resin copolymer monomer initiator feed list
[0029]
[0030] a. Add the monomers in Table 1 except coconut oleic acid to the four-neck flask with stirring, thermometer and rectification column, control the temperature of the kettle not to exceed 230°C, the temperature of the column is 95-100°C, and react for 2-4hrs Weigh the amount of water, the acid value is less than 5mgKOH / g, add coconut oil acid, continue the reaction for 1-1.5hr, the acid value is less than 5mgKOH / g, and cool down to 100°C.
[0031] b. Add 150g propylene glycol methyl ether and 80g ethylene glycol monobutyl ether, and control the temperature to 90-100°C;
[0032] c. At 90-100°C, methyl methacrylate (MMA), butyl acrylate (BA), lauryl methacrylate (LMA), glycidyl methacrylate (GMA), isobornyl methacrylate Mix monomers such as ester (IBOMA) and azobisisobutyronitrile (AIBN) evenly,...
Embodiment 2
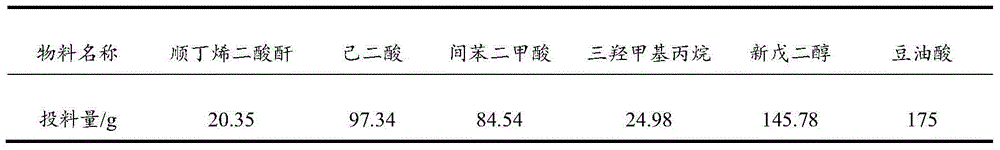
[0036] Table 3 Polyacid and polyol feed list for partial synthesis of polyester
[0037]
[0038] Table 4. Acrylic resin copolymer monomer initiator feed list
[0039]
[0040] a. Add the monomers in Table 1 except soybean oil to a four-neck flask equipped with stirring, thermometer and rectification column, control the temperature of the kettle not to exceed 230°C, and the temperature of the column at 95-100°C, and weigh after 2-4hrs of reaction The amount of water output, the measured acid value is less than 5mgKOH / g, add soy oil, continue the reaction for 1-1.5hr, the measured acid value is less than 5mgKOH / g, and cool down to 100°C.
[0041] b. At 100°C, methyl methacrylate (MMA), butyl acrylate (BA), butyl methacrylate (BMA), glycidyl methacrylate (GMA), isobornyl methacrylate ( IBOMA) and other monomers and benzoyl peroxide (BPO) were mixed evenly, and added dropwise into a four-necked bottle, and the dropwise addition time was 2-2.5hr. After the dropwise addition...
Embodiment 3
[0045] Table 5 Polyacid and polyol feed list for partial synthesis of polyester
[0046]
[0047] Table 6. Acrylic resin copolymer monomer initiator feed list
[0048]
[0049] a. Add the monomers in Table 1 except soybean oil to a four-neck flask equipped with stirring, thermometer and rectification column, control the temperature of the kettle not to exceed 230°C, and the temperature of the column at 95-100°C, and weigh after 2-4hrs of reaction The amount of water produced is less than 5mgKOH / g as measured by the acid value. Add soy oil and continue the reaction for 1-1.5hr. The measured acid value is less than 5mgKOH / g, and the temperature is lowered to 110°C.
[0050] b. At 110°C, methyl methacrylate (MMA), butyl acrylate (BA), lauryl methacrylate (LMA), glycidyl methacrylate (GMA), isobornyl methacrylate ( IBOMA) and other monomers and tert-butyl peroxybenzoate (TBPB) were mixed evenly, and added dropwise into a three-necked bottle for 2 to 2.5 hours. After the add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com