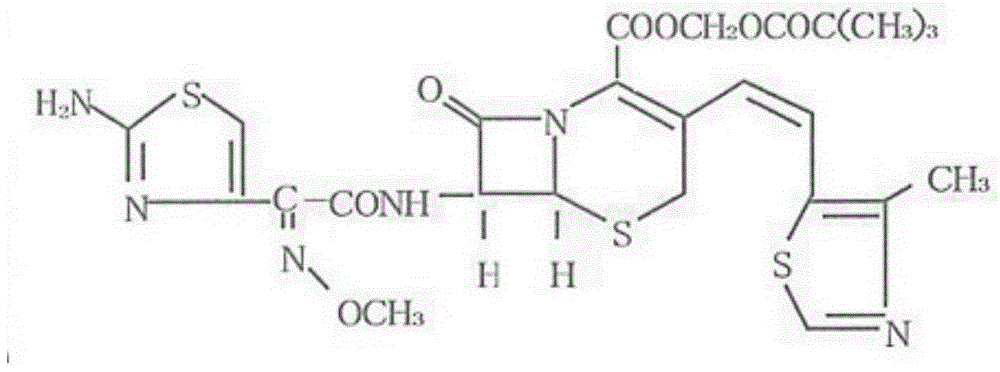
Cefditoren pivoxil tablets
A technology of cefditoren pivoxil and cefditoren, which is applied in the field of medicine, can solve the problems of high water content, long drying time, and limited improvement of drug dissolution rate, and achieve the effect of fast dissolution rate and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]
[0022] Preparation Process:
[0023] Cefditoren pivoxil was dissolved in diethylene glycol monoethyl ether and 40g ethanol, added hydroxypropyl cellulose, stirred to dissolve, then added the prescribed amount of fumed silica for adsorption, and then mixed with mannitol and cross-linked carboxylate Sodium methylcellulose and magnesium stearate are uniformly mixed and compressed by direct compression technology.
Embodiment 2
[0025]
[0026] Preparation Process:
[0027] Cefditoren pivoxil is dissolved in diethylene glycol monoethyl ether and 40g ethanol, adds hydroxypropyl cellulose, stirs and makes dissolving, then adds the fumed silicon dioxide adsorption of recipe quantity, then with pregelatinized starch, low The hydroxypropyl cellulose and magnesium stearate are mixed evenly and compressed by direct compression technology.
Embodiment 3
[0029]
[0030] Preparation Process:
[0031] Cefditoren pivoxil was dissolved in diethylene glycol monoethyl ether and 40g ethanol, added hydroxypropyl cellulose, stirred to dissolve, then added the prescribed amount of fumed silica for adsorption, and then mixed with mannitol and cross-linked carboxylate Sodium methylcellulose and magnesium stearate are uniformly mixed and compressed by direct compression technology.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com