Molecular glue-based fluorescently labeled nucleotides and their use in dna sequencing
A fluorescent labeling, nucleotide technology, applied in the fields of chemical synthesis and biochemistry, which can solve problems such as poor selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] This embodiment relates to a fluorescently labeled nucleotide based on molecular glue, that is, a reversible terminal whose structural formula is the following formula (II):
[0085]
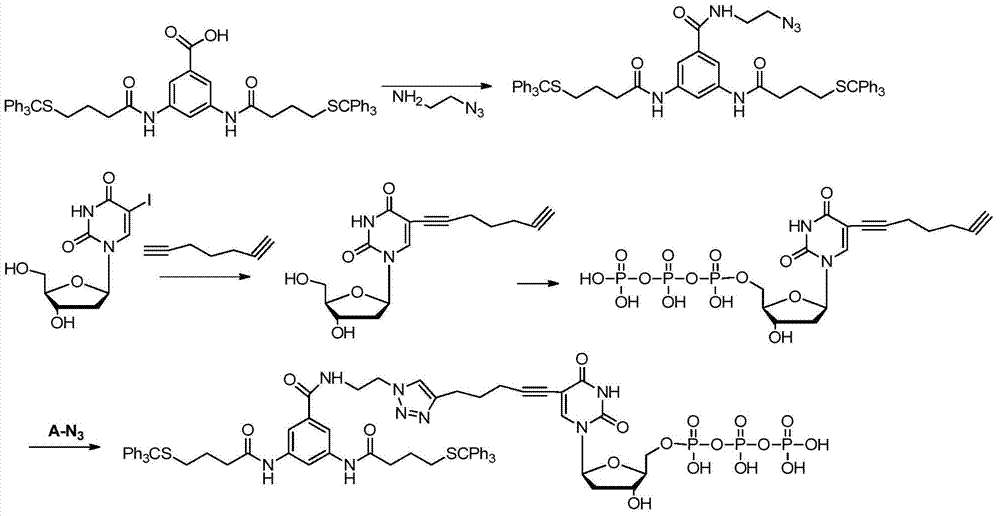
[0086] The corresponding synthetic route is as Image 6 Shown; specifically include the following steps:
[0087] 1 compound dUTP (AP 3 )-A synthesis
[0088] 1.1dUTP (AP 3 )Synthesis:
[0089]
[0090] 1.1.1 Compound trifluoroethylpropynylamine F 2 Synthesis
[0091] Methyl trifluoroacetate reacts with propargylamine in an organic solvent to obtain compound F 2 , specifically: add 60ml of methanol to a single-necked bottle, stir under an ice-water bath, add propargylamine (60mmol, 3.3042g), stir for 15 minutes and then slowly add methyl trifluoroacetate (86.7mmol, 11.0957g) for 10 minutes Afterwards, the ice-water bath was removed, and the reaction was carried out at room temperature for 24 hours. The reaction was monitored with a TLC plate, PE:EA=8:1, baking plate, Rf=0.5,...
Embodiment 2
[0129] This embodiment relates to a fluorescently labeled nucleotide based on molecular glue, that is, a reversible terminal whose structural formula is the following formula (III):
[0130]
[0131] Specifically include the following steps:
[0132] 1 Synthesis of compound dUTP-A-click
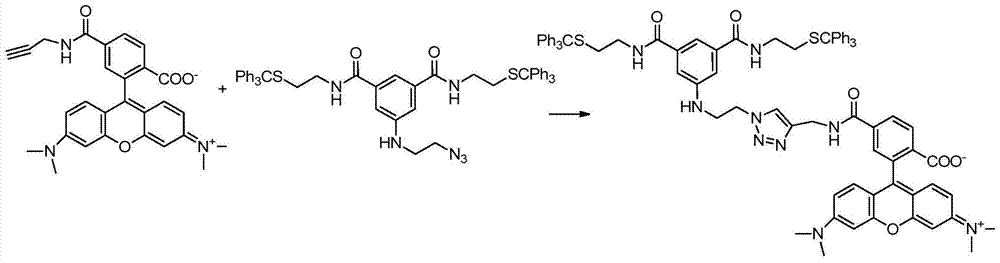
[0133] 1.1 Compounds A-N 3 Synthesis
[0134] Compound A-N 3 The synthetic route of figure 2 Shown: Under basic conditions, compound A is subjected to amidation reaction with 2-azidoethylamine whose terminal is an amino group to obtain compound A-N 3 ;
[0135] The steps are as follows: Weigh compound A (0.841g, 0.1mmol) and dissolve 5ml DMF in a 10ml single-necked flask, add NMM (N-methylmorpholine) (200μL, 2mmol), HATU (2- (7-azobenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate) (0.57g, 1.5mmol) was activated for 30min, and 2-azidoethylamine ( 129mg, 1.5mmol) stirred for 1h, warmed up to room temperature and reacted for 8h, stopped the reaction, added an appropriate...
Embodiment 3
[0153] This embodiment relates to a fluorescently labeled nucleotide based on molecular glue, that is, the structural formula is a reversible terminal shown in the following formula (IV):
[0154]
[0155] Its synthetic concrete steps are as follows:
[0156] 1 compound dUTP (AP 3 )-A synthesis
[0157] The synthesis of dUTP(AP3)-A refers to Example 1.
[0158] 2 Synthesis of compound TAMRA-B-click
[0159] 2.1 Compounds B-N 3 Synthesis
[0160] B-N 3 The synthetic route of is as follows:
[0161]
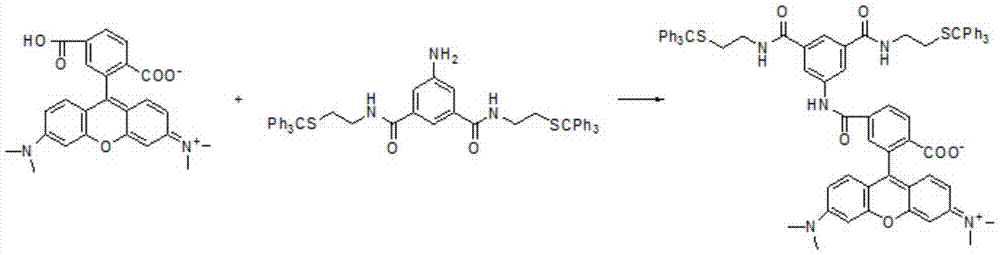
[0162] The steps are as follows: Weigh bromoethanediazide (0.3g, 2mmol), B (1.14g, 1.5mmol) in a dry single-necked flask of 10ml, add NMM (N-methylmethanol) under ice-water bath and nitrogen protection phenoline) (200μL, 2mmol), dry DMF10ml, reflux at 120°C for 18h, stop the reaction, extract the reaction solution with dichloromethane, rotary evaporate to obtain 1.3g of crude product, column chromatography 1.0mg, and obtain B-N 3 , yield 82%. 1 H NMR (500MHz, CDCl 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com