Cleviprex fat emulsion concentrated solution, preparation method and application thereof
A technology of clevidipine butyrate and fat emulsion, which can be used in emulsion delivery, cardiovascular system diseases, drug combination and other directions, and can solve problems such as hidden dangers of drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
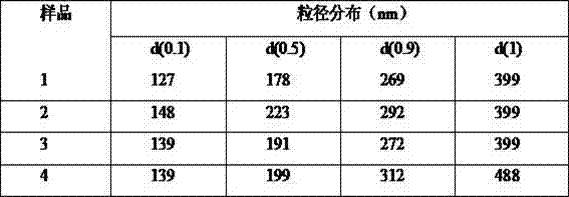
[0163] Embodiment 1: Preparation of clevidipine butyrate fat emulsion concentrate sample 1
[0164] Its composition is as follows:
[0165] Clevidipine Butyrate 0.35g
[0166] soybean oil 5 grams
[0167] Medium Chain Fatty Acid Triglycerides (Crodamol GTCC) 65g
[0168] 1, 2-Propanediol 8.5g
[0169] Soy lecithin (Epikuron 170, Degussa) 8 grams
[0170] Tween 80 4.3 g
[0171] Polyethylene glycol lauryl hydroxystearate (Solutol HS 15, BASF) 8.5 g
[0172] Vitamin E 0.35g
[0173] The preparation method of this clevidipine butyrate fat emulsion concentrate is as follows:
[0174] 1) At 45°C, mix the above-mentioned weight of soybean lecithin, soybean oil and medium-chain fatty acid triglycerides, and stir at 8000rpm until a transparent and clear solution is formed;
[0175] 2) At 20°C, add the above weight of clevidipine butyrate to the product of 1), and keep stirring at 200rpm until the whole system is transparent and clear;
[0176] 3) At 25°C, add the above-m...
Embodiment 2
[0178] Embodiment 2: Preparation of clevidipine butyrate fat emulsion concentrate sample 2
[0179] Its composition is as follows:
[0180] Clevidipine Butyrate 0.25g
[0181] 30.5g soybean oil
[0182] Glyceryl caprylate (Miglyol 812, SASOL) 30.5g
[0183] 1, 2-Propanediol 7.3g
[0184] Soy Lecithin (Epikuron 170, Degussa) 11.5g
[0185] Polyglyceryl Oleate 4g
[0186] Polyethylene glycol lauryl hydroxystearate (Solutol HS 15, BASF) 11.5 g
[0187] Tween 80 4g
[0188] Vitamin E 0.3g
[0189] Oleic acid 0.15g
[0190] The preparation method of this clevidipine butyrate fat emulsion concentrate is as follows:
[0191] 1) At 20°C, mix the above-mentioned weight of soybean lecithin, polyglycerol oleate, caprylic capric glyceride and soybean oil, and stir at 20,000 rpm until a transparent and clear solution is formed;
[0192] 2) At 45°C, add the above weight of clevidipine butyrate to the product of 1), and keep stirring at 1000rpm until the whole system is trans...
Embodiment 3
[0195] Embodiment 3: Preparation of clevidipine butyrate fat emulsion concentrate sample 3
[0196] Its composition is as follows:
[0197] Clevidipine Butyrate 0.3g
[0198] soybean oil 50g
[0199] 1,2-Propanediol 9.5g
[0200] Soy Lecithin (Epikuron 170, Degussa) 12.5g
[0201] Polyglyceryl Oleate 3 g
[0202] Polyethylene glycol lauryl hydroxystearate (Solutol HS 15, BASF) 13.5 g
[0203] Tween 80 10.5g
[0204] Vitamin E 0.35g
[0205] Oleic acid 0.35g
[0206] The preparation method of this clevidipine butyrate fat emulsion concentrate is as follows:
[0207] 1) At 25°C, mix the above-mentioned weight of soybean lecithin, polyglycerol oleate and soybean oil, and stir at 2000rpm until a transparent and clear solution is formed;
[0208] 2) At 30°C, add the above weight of clevidipine butyrate to the product of 1), and keep stirring at 2000rpm until the whole system is transparent and clear;
[0209] 3) At 20°C, add the above-mentioned weight of 1,2-propane...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com