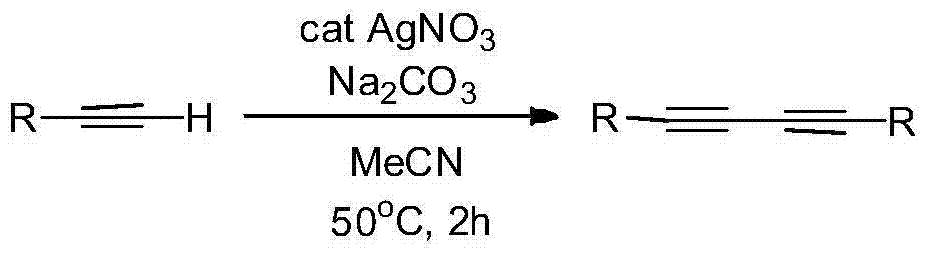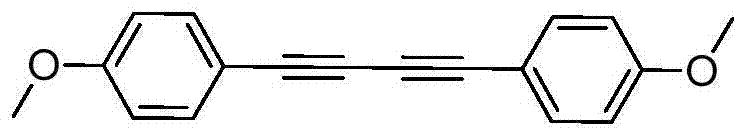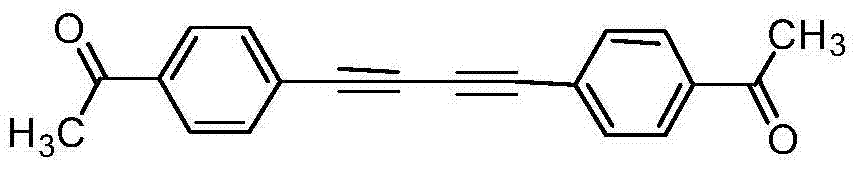Preparation method of symmetric 1,4-disubstituted-1,3-diacetylene
A disubstituted, diacetylene technology, applied in the field of chemical synthesis, can solve the problems of high price, large environmental pollution, complicated operation, etc., and achieve the effect of high practicability and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 1 mmol of p-methoxyphenylacetylene, 0.01 mmol of silver nitrate and 0.5 mmol of sodium carbonate into a round bottom flask filled with 1 ml of acetonitrile, and stir for 2 hours at 50°C. After the reaction was completed, the solid was filtered out, and the solvent was spin-dried, and the product was obtained by column separation with a yield of 94%.
[0027]
[0028] 1 H NMR (400MHz, CDCl 3 , TMS) δ7.46(d, J=8.4Hz, 4H), 6.85(d, J=8.8Hz, 4H), 3.81(s, 6H). 13 C NMR (100MHz, CDCl 3 ): δ160.3, 134.1, 114.2, 113.8, 81.3, 72.8, 55.3. HRMS calcd for C 18 h 10 o 2 : 262.0994, found: 262.0997.
Embodiment 2
[0030] Add 1mmol of p-acetylphenylacetylene, 0.01mmol of silver nitrate and 0.5mmol of sodium carbonate into a round bottom flask containing 1ml of acetonitrile, and stir for 2 hours at 50°C. After the reaction was completed, the solid was filtered out, and the solvent was spin-dried, and the product was obtained by column separation, with a yield of 90%.
[0031]
[0032] 1 H NMR (CDCl 3 , 400MHz) δ7.94(d, J=8.4Hz, 4H) 7.62(d, J=8.4Hz, 4H), 2.62(s, 6H). 13 C NMR (100MHz, CDCl 3 ): δ196.7, 137.1, 132.7, 128.3, 126.0, 81.9, 76.6, 26.6. HRMS calcd for C 20 h 14 o 2 :286.0994.found: 286.0993.
Embodiment 3
[0034] Add 1mmol of 1-naphthyneacetylene, 0.01mmol of silver nitrate and 0.5mmol of sodium carbonate into a round bottom flask filled with 1ml of acetonitrile, and stir at 50°C for 2 hours. After the reaction was completed, the solid was filtered out, and the solvent was spin-dried, and the product was obtained by column separation with a yield of 89%.
[0035]
[0036] 1 H NMR (CDCl 3 , 400MHz) δ8.44(d, J=8.0Hz, 2H), 7.82-7.92(m, 6H), 7.43-7.68(m, 6H). 13 C NMR (100MHz, CDCl 3 ): δ133.9, 133.0, 132.1, 129.6, 128.3, 127.2, 126.6, 126.1, 125.1, 119.5, 81.0, 78.8. HRMS calcd for C 24 h 14 : 302.11096, found: 302.1092.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com