Epoxy cardanol-based glycidyl ether as well as preparation method and application thereof
A technology based on glycidyl ether and cardanol, which is applied in the field of epoxy plasticizer preparation, can solve the problems of poor compatibility, limited range of use of plasticizers, single variety, etc., and achieve good resin compatibility and excellent Plasticizing effect and environmental protection performance, the effect of simplifying the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] A kind of preparation method of epoxy cardanol base glycidyl ether, the steps are:
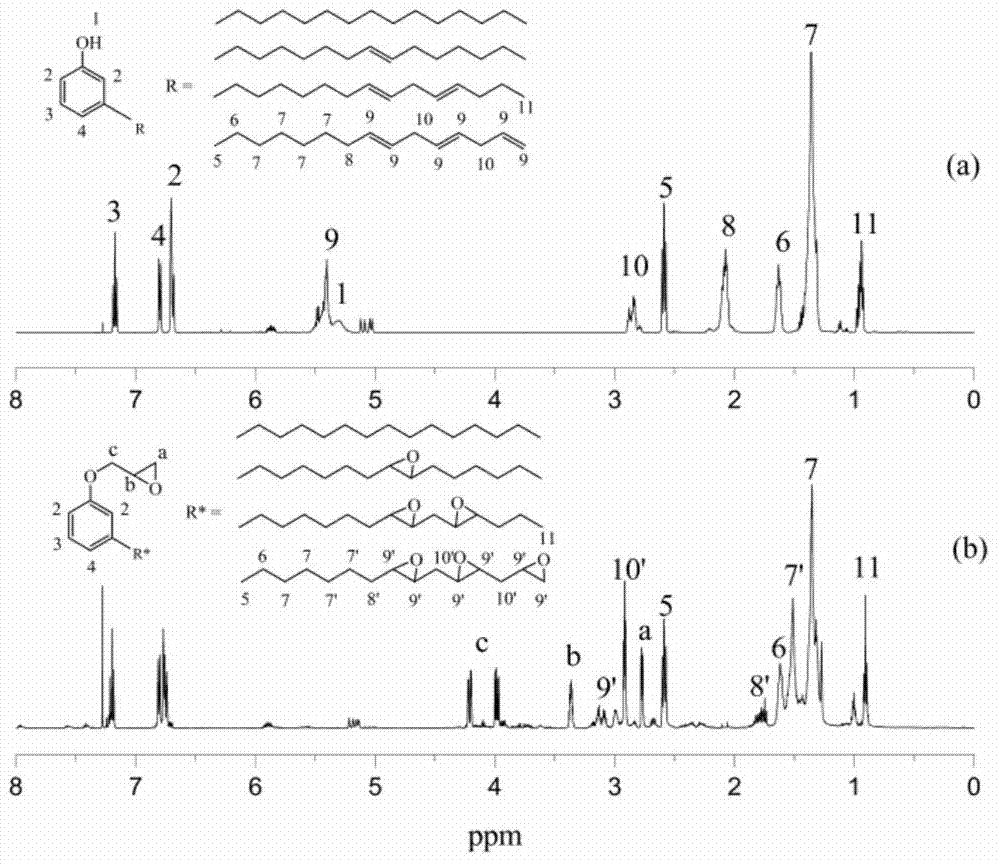
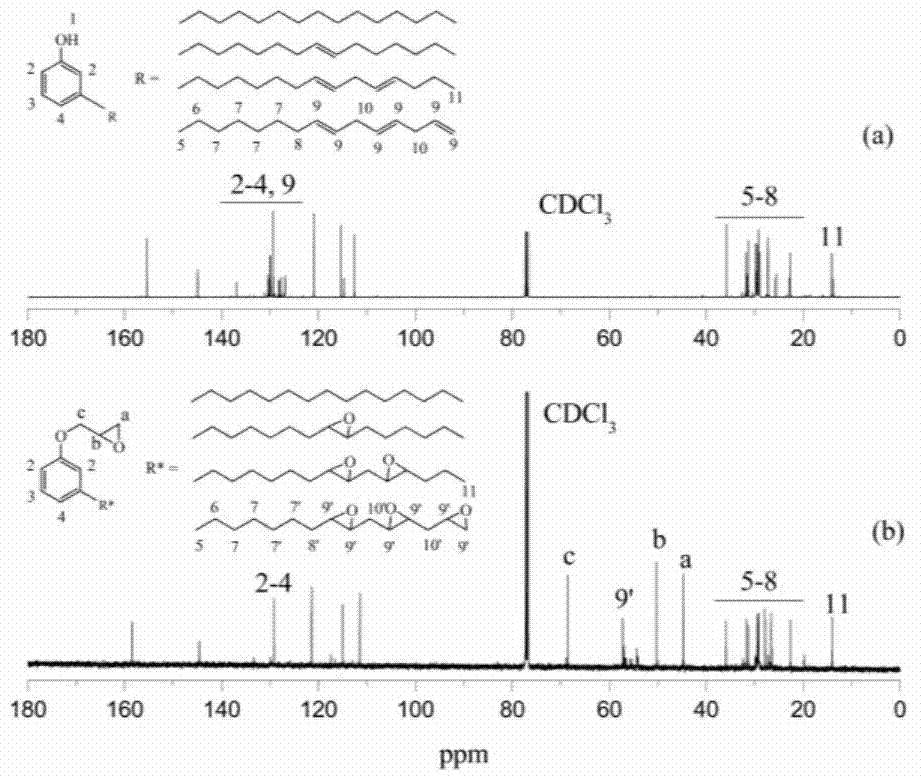
[0029] (1) 50-100g of cardanol, 100-200g of epichlorohydrin and an appropriate amount of ring-opening addition catalyst (accounting for 1-2% of the mass of cardanol) are carried out at 90-120°C for ring-opening addition reaction 2 ~4h; continue to add 5-15g of strong base and conduct ring closure reaction at 50~70°C for 3~5h; then filter and vacuum distillation to recover excess epichlorohydrin to obtain the intermediate product cardanol glycidyl ether. (2) Weigh 10-20g of cardanol-based glycidyl ether, 0.5-1.5g of organic acid and an appropriate amount of catalyst (accounting for 2-3% of the mass of cardanol), add 3-10g of hydrogen peroxide dropwise at 50°C, and add 3-10g of hydrogen peroxide at 60-70°C After reacting for 3 to 5 hours, the crude product of epoxy cardanol glycidyl ether is obtained, and after static stratification, neutralization and dehydration treatment, an environmen...
Embodiment 1
[0040] (1) Weigh 50g of cardanol, 122g of epichlorohydrin and 0.9g of benzyltriethylammonium chloride, add them into a three-necked flask equipped with a condenser tube, a stirring rod and a thermometer, and react at 98°C for 3h; After the end, cool to room temperature, add 6.6g of solid sodium hydroxide, and react at 60°C for 3 hours; after filtration and vacuum distillation, excess epichlorohydrin is recovered to obtain the intermediate product cardanyl glycidyl ether.
[0041] (2) Weigh 20g of cardanol glycidyl ether, 1.2g of formic acid and 0.4g of p-toluenesulfonic acid, add them into a three-necked flask equipped with a condenser, a stirring rod and a thermometer, stir and heat up to 50°C, and add 7.6g of hydrogen peroxide dropwise (50%), dripped in about 0.5h, raised the temperature to 65°C for 4h; after the reaction, the crude product was left to separate to obtain the organic phase, and the mass fraction of 50°C was 5% sodium bicarbonate solution and 50°C Wash with de...
Embodiment 2
[0043] (1) Weigh 50g of cardanol, 122g of epichlorohydrin and 1.0g of tetramethylammonium chloride, add them into a three-necked flask equipped with a condenser tube, a stirring rod and a thermometer, and react at 110°C for 2h; , cooled to room temperature, added 6.6g of solid potassium hydroxide, and reacted at 60°C for 3h; after filtration and vacuum distillation, excess epichlorohydrin was recovered to obtain the intermediate product cardanyl glycidyl ether.
[0044] (2) Weigh 20g of cardanol glycidyl ether, 1.2g of acetic acid and 0.5g of p-toluenesulfonic acid, add them into a three-necked flask equipped with a condenser, a stirring rod and a thermometer, stir and heat up to 50°C, and add 8.5g of hydrogen peroxide dropwise (50%), dripped in about 0.5h, raised the temperature to 65°C for 3h; after the reaction, the crude product was left to separate to obtain the organic phase, and the mass fraction of 50°C was 5% sodium bicarbonate solution and 50°C Wash with deionized wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com