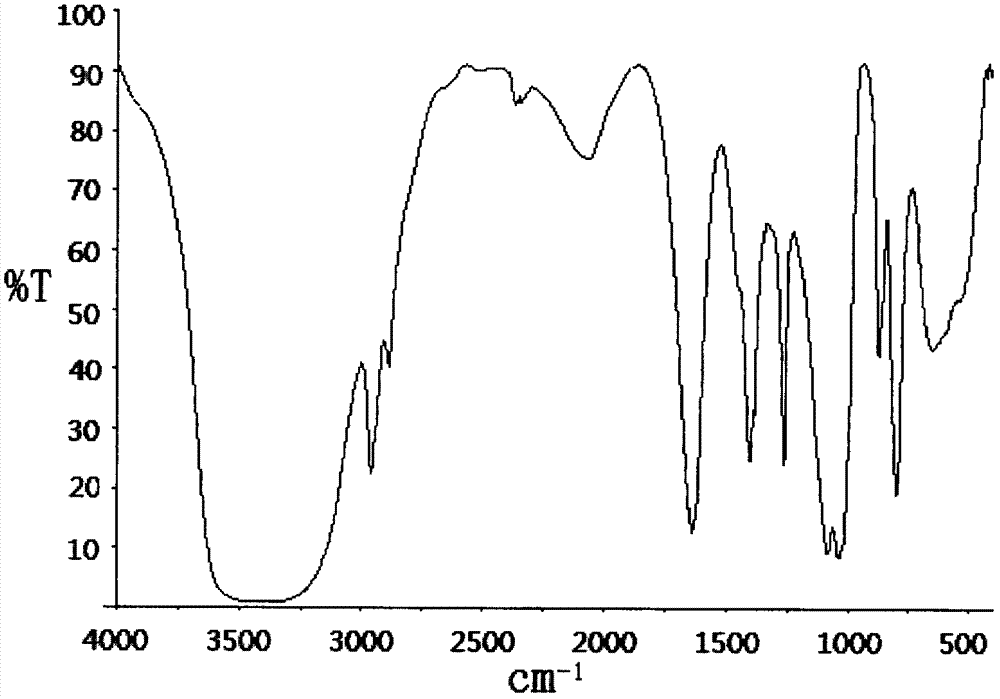
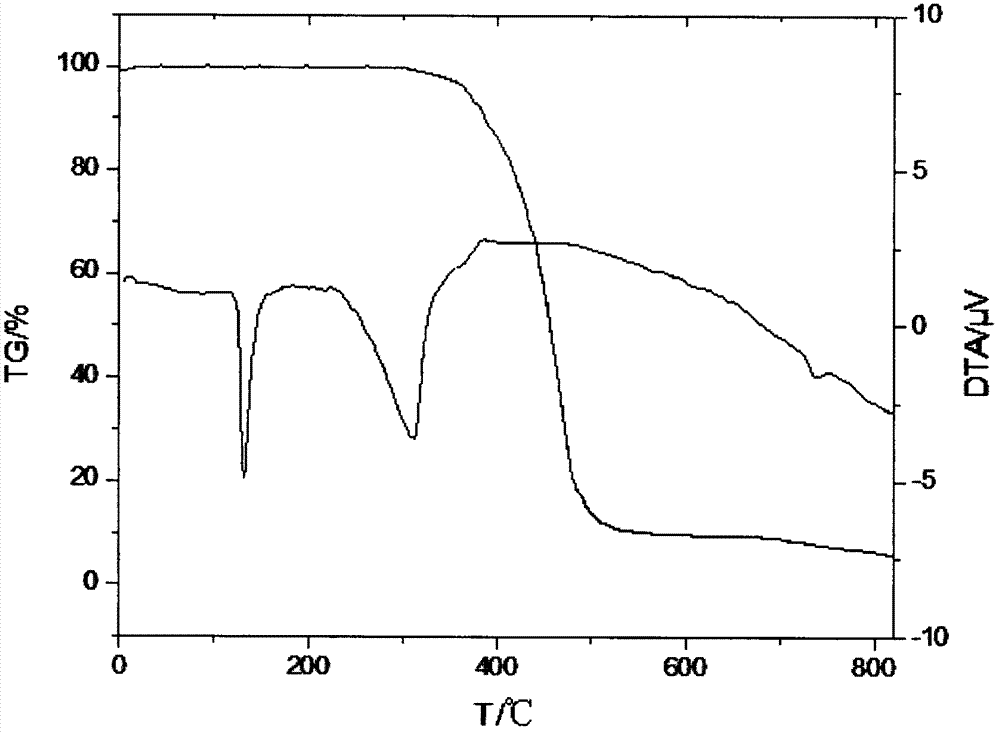
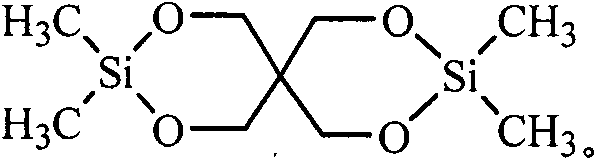Dimethyl silicate pentaerythritol ester compound as fire retardant and preparation method thereof
A technology of pentaerythritol dimethylsilicate and pentaerythritol, which is applied in chemical instruments and methods, compounds of elements of Group 4/14 of the periodic table, organic chemistry, etc., can solve the limitation of halogen-based flame retardants, secondary hazards, A large amount of hydrogen halide gas and other problems, to achieve the effect of good compatibility, low cost, good application and development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 In a 100ml four-neck flask equipped with a stirrer, a thermometer, a high-efficiency reflux condenser and a drying tube and a hydrogen chloride absorption device at the upper mouth of the condenser, add 30ml of ethylene glycol dimethyl ether and 6.8g (0.05mol ) pentaerythritol, stirred and heated to 75°C to fully dissolve the pentaerythritol, then cooled to 40°C, added dropwise 12.9g (0.1mol) of dimethyldichlorosilane, and the dropping process controlled the temperature not to exceed 50°C. Insulate at 50°C for 2 hours, then raise the temperature to 70°C, heat for 8 hours, stir and cool to 30°C, filter with suction, recover the solvent, wash the filter cake with water to remove the attached free acid, and dry the filter cake to obtain a white solid dimethyl Pentaerythritol silicate, product yield 92.6%.
Embodiment 2
[0027] Example 2 Add 40ml of acetonitrile and 6.8g (0.05mol) of pentaerythritol in a 100ml four-neck flask equipped with a stirrer, a thermometer, a high-efficiency reflux condenser and a drying tube and a hydrogen chloride absorption device at the upper mouth of the condenser, and stir and heat To 75°C, fully dissolve pentaerythritol, then lower the temperature to 40°C, add 12.9g (0.1mol) dimethyldichlorosilane dropwise, control the temperature during the dropwise addition to not exceed 50°C, after the drop, raise the temperature to 60°C, Insulate for 2 hours, then heat up to 80°C, heat for 6 hours, stir and cool to 30°C, filter with suction, recover the solvent, wash the filter cake with water to remove the attached free acid, dry the filter cake to obtain white solid pentaerythritol dimethyl silicate ester, the product yield is 93.5%.
Embodiment 3
[0028] Example 3 Add 50ml of dichloroethane and 6.8g (0.05mol) of pentaerythritol in a 100ml four-necked flask equipped with a stirrer, a thermometer, a high-efficiency reflux condenser and a drying tube and a hydrogen chloride absorption device at the upper mouth of the condenser , stirred and heated to 75°C to fully dissolve the pentaerythritol, then lower the temperature to 40°C, add 12.9g (0.1mol) dimethyldichlorosilane dropwise, and control the temperature during the dropwise addition to not exceed 50°C. 60°C, keep warm for 2h, then raise the temperature to 82°C, keep warm for 6h, stir and cool to 30°C, filter with suction, recover the solvent, wash the filter cake with water to remove the attached free acid, dry the filter cake to obtain a white solid dimethyl Pentaerythritol silicate, product yield 94.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com