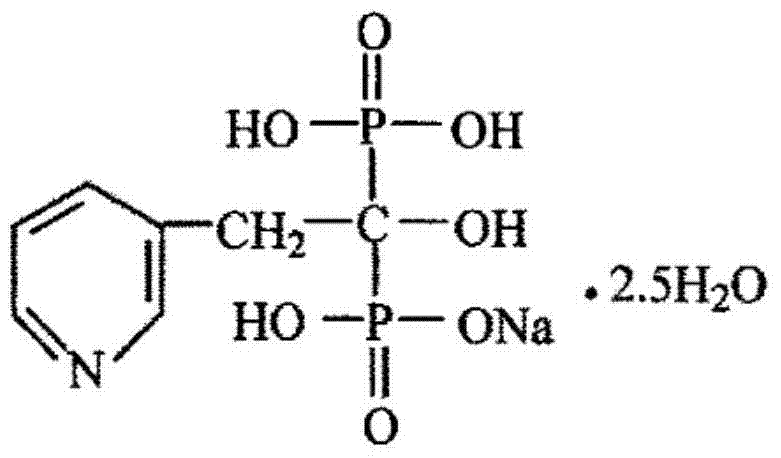
Composition for risedronate sodium coated tablet and preparation method thereof
A technology of risedronate sodium and coated tablets, which is applied in the direction of drug combination, pharmaceutical formula, drug delivery, etc., can solve the problems of high substance content, rare research, low drug safety, etc. The effect of safety improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 risedronate sodium coated tablet (5mg / tablet) of the present invention
[0031] prescription:
[0032] Chip layer:
[0033]
[0034] Coating layer:
[0035] Opadry Gastric Film Coating Premix 3g,
[0036] Purified water 30g.
[0037] Preparation:
[0038] Dissolve the prescribed amount of risedronate sodium, hypromellose, and polyethylene glycol in water, spray-dry to form a coating powder, add the prescribed amount of chitosan and other excipients, mix well, press into tablets, and coat (the weight gain of the coating layer is 2% of the weight of the tablet core layer), to get final product.
Embodiment 2
[0039] Embodiment 2 risedronate sodium coated tablet (35mg / tablet) of the present invention
[0040] prescription:
[0041] Chip layer:
[0042]
[0043]
[0044] Coating layer:
[0045] Opadry Gastric Film Coating Premix 6g,
[0046] 85% ethanol 120g.
[0047] Preparation:
[0048] Dissolve the prescribed amount of risedronate sodium, hypromellose, and polyethylene glycol in water, spray-dry to form a coating powder, add the prescribed amount of chitosan and other excipients, mix well, press into tablets, and coat (the weight gain of the coating layer is 3% of the weight of the tablet core layer), to get final product.
Embodiment 3
[0049] Embodiment 3 risedronate sodium coated tablet (70mg / tablet) of the present invention
[0050] prescription:
[0051] Chip layer:
[0052]
[0053] Coating layer:
[0054] Opadry Gastric Film Coating Premix 12g,
[0055] Purified water 100g.
[0056] Preparation:
[0057] Dissolve the prescribed amount of risedronate sodium, hypromellose, and polyethylene glycol in water, spray-dry to form a coating powder, add the prescribed amount of chitosan and other excipients, mix well, press into tablets, and coat (the weight gain of the coating layer is 4% of the tablet core layer weight), to get final product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com