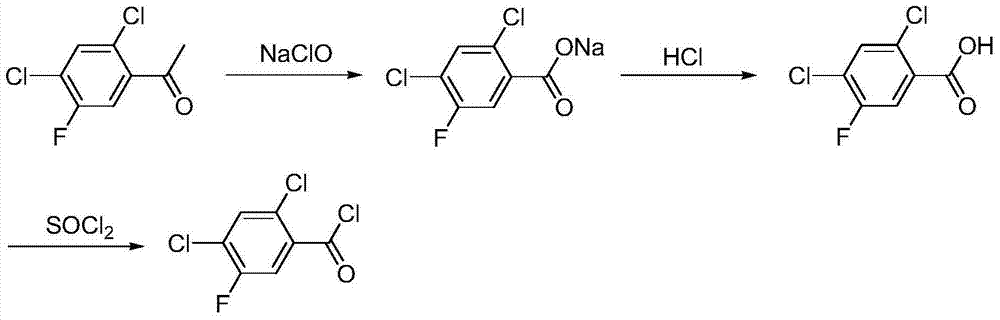
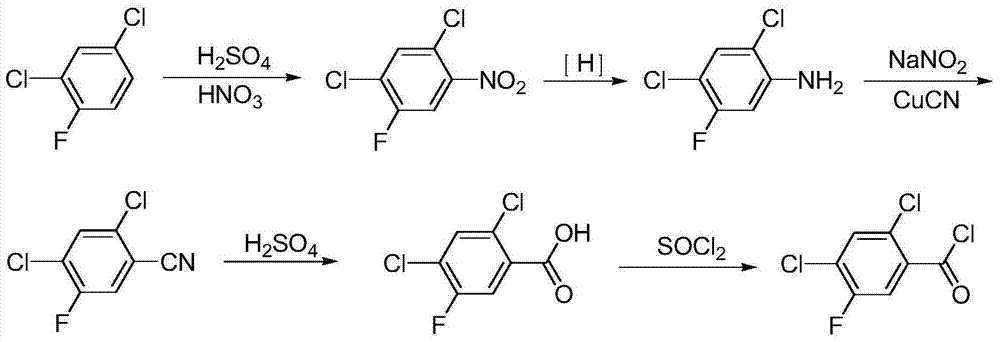
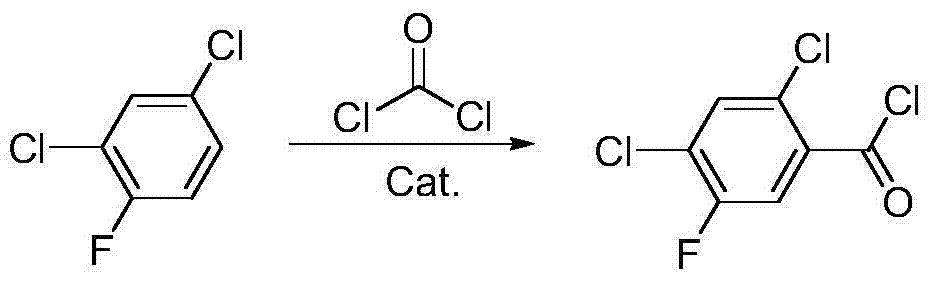
Method for synthesizing 2,4-dichloro-5-fluorobenzoyl
A technology for the synthesis of fluorobenzoyl chloride and its synthesis method, which is applied in the field of synthesis of 2,4-dichloro-5-fluorobenzoyl chloride, and achieves the effects of less pollutant discharge, good development prospects, and convenient recycling and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0032] 200 mL of SmCl with a concentration of 0.5 mol / L 3 and 100 mL of ZrOCl with a concentration of 0.5 mol / L 2 Place in a reaction vessel, add 200 mL of AlCl with a concentration of 0.5 mol / L under vigorous stirring 3 , and then slowly add concentrated ammonia water until the pH of the system is 9-10; after aging the reaction system at -18°C for 24 hours, suction filter and wash until there is no Cl - After detection, the filter cake was dried at 120°C, impregnated with 500mL ammonium persulfate solution with a concentration of 1mol / L for 4 hours, filtered, then dried, activated at 600°C for 3h to obtain 38g of solid acid catalyst S 2 o 8 2- / Sm 2 o 3 -ZrO 2 -Al 2 o 3 (ie catalyst Cat.1).
Embodiment 2-1
[0035] step 1)
[0036] Add 16.5 g (0.1 mol) of 2,4-dichlorofluorobenzene, CCl 4 15.4g (0.1mol), catalyst Cat. 10.83g and AlCl 3 33.3g (0.25mol), heat up to 40°C and stir for 4 hours. The hydrogen chloride produced by the reaction is absorbed by falling film. After the reaction, slowly inject the reactant into 50ml of distilled water, filter and recover the catalyst Cat.1, and the filtrate is separated. , the organic layer was washed with water, dried, and then distilled under reduced pressure to obtain 26.9 g of 2,4-dichloro-5-fluoro-(trichloromethyl)benzene with a yield of 95.2%.
[0037] step (2)
[0038] Add 26.9 g (0.095 mol) of 2,4-dichloro-5-fluoro-(trichloromethyl)benzene prepared in step (1) into the reaction flask, and then add FeCl 3 0.17g, heat up to 130°C, slowly add 1.8g (0.1mol) of distilled water dropwise under stirring conditions, drop it over 10 hours, and absorb the hydrogen chloride generated by the reaction with water. After the reaction, the reaction s...
Embodiment 2-2
[0040] step 1)
[0041] Add 16.5 g (0.1 mol) of 2,4-dichlorofluorobenzene, CCl 4 7.7g (0.05mol), catalyst Cat. 10.165g and AlCl 3 13.3g (0.1mol), heat up to 20°C and stir for 5 hours to react. The hydrogen chloride produced by the reaction is absorbed by falling film. After the reaction, slowly inject the reactant into 50ml of distilled water, filter and recover the catalyst Cat.1, and separate the filtrate , the organic layer was washed with water, dried, and then distilled under reduced pressure to obtain 13.1 g of 2,4-dichloro-5-fluoro-(trichloromethyl)benzene with a yield of 92.7%.
[0042] step (2)
[0043] Take 2,4-dichloro-5-fluoro-(trichloromethyl)benzene 28.3g (0.1mol) prepared by step (1) and add it to the reaction flask, then add FeCl 3 0.085g, heat up to 100°C, slowly add 1.8g (0.1mol) of distilled water dropwise under stirring conditions, drop it over 10 hours, absorb the hydrogen chloride generated by the reaction with water, after the reaction, the reaction s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com