Epoxide hydrolase mutant, engineering bacteria and application of epoxide hydrolase mutant
An epoxide and hydrolase technology, which is applied in the field of bioengineering technology, can solve the problems of limited application potential and low enantioselectivity, and achieves good industrial application prospects, high catalytic activity, and improved enantioselectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of epoxide hydrolase mutant
[0032] Site-directed saturation mutagenesis and site-directed mutagenesis refer to the protocol described in (Current Protocols in Protein Science 2011, 26.6.1-26.6.10; Anal. Biochem. 2008, 375: 376-378). First, design the mutation primers containing the mutation points, as shown in Table 1. The epoxide hydrolase (GenBank no.JX467176) derived from Agromyces mediolanus CCTCC M 2012299 consists of 288 amino acid residues (the amino acid sequence is shown in SEQ ID NO.13), and the expression vector pET28-AmEH has been successfully constructed. The plasmid pET28a -AmEH was used as template for whole plasmid amplification. The PCR system is: 5×PS Buffer 10 μl, dNTP (2.5mM each) 4 μl, mutant primers F and R 0.5 μl each, plasmid pET28-AmEH 0.5 μl, PrimeSTAR DNA polymerase 0.5 μl, and water to 50 μl. PCR conditions were pre-denaturation at 98°C for 2 minutes, 27 cycles: 98°C for 10s, 65°C for 10s, 72°C for 7min, and ...
Embodiment 2
[0035] Example 2: Induced Expression and Purification of Epoxide Hydrolase Parents and Mutants
[0036] 1. Expression of Epoxide Hydrolase Parents and Mutants
[0037] The starting strain E.coli BL21(DE3) / pET28-AmEH of Example 1 and the mutant strain in Example 1 were respectively inoculated into LB liquid medium containing 50mg / L kanamycin, cultivated at 37°C for 12h, and then Inoculate into fresh LB liquid culture medium containing 50mg / L kanamycin with 1% inoculum size (v / v), cultivate to bacterium concentration OD 600 About 0.6, then add IPTG with a final concentration of 0.1mM to the LB liquid medium, induce culture at 28°C for 10h, then centrifuge at 8000g for 10min at 4°C, and collect the bacterial cells containing recombinant epoxide hydrolase, which can be used for enzyme production Purification and biocatalytic preparation of (S)-ECH.
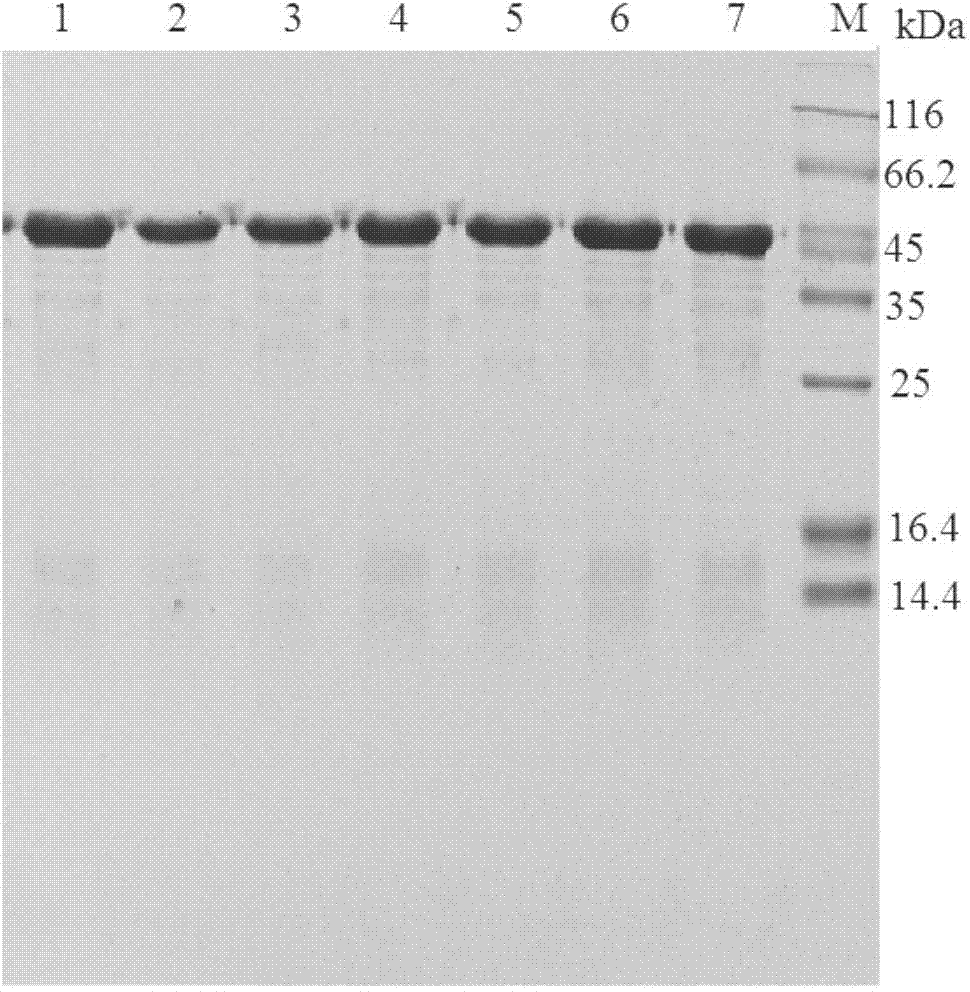
[0038] 2. Purification of Epoxide Hydrolase Parents and Mutants
[0039] After the epoxide hydrolase bacterial cells were ultraso...
Embodiment 3
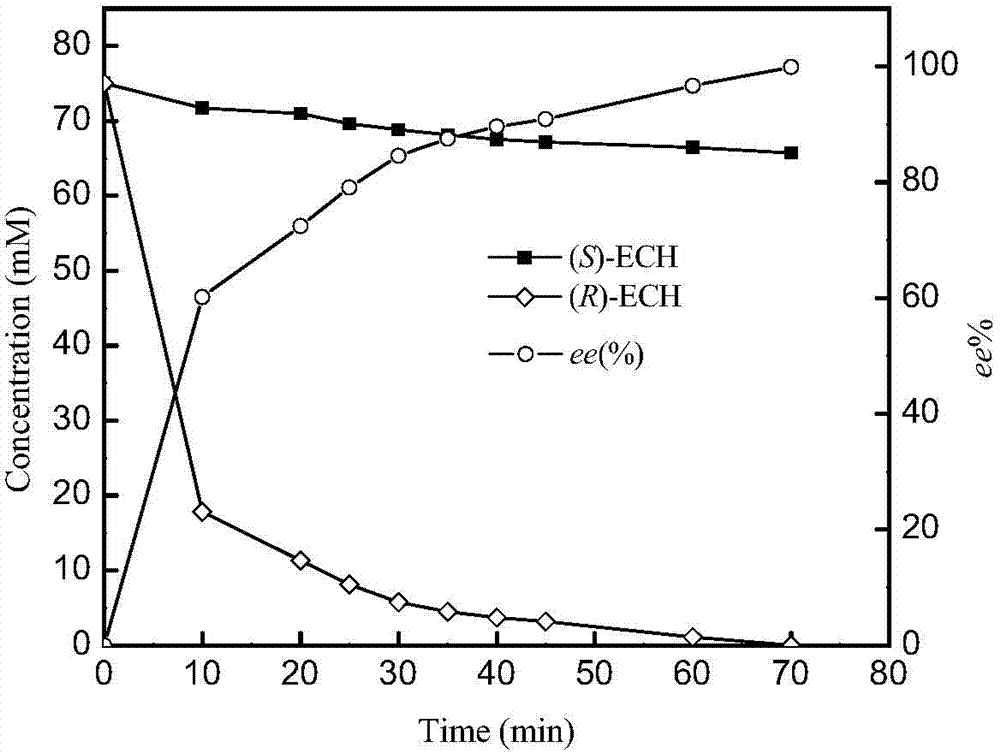
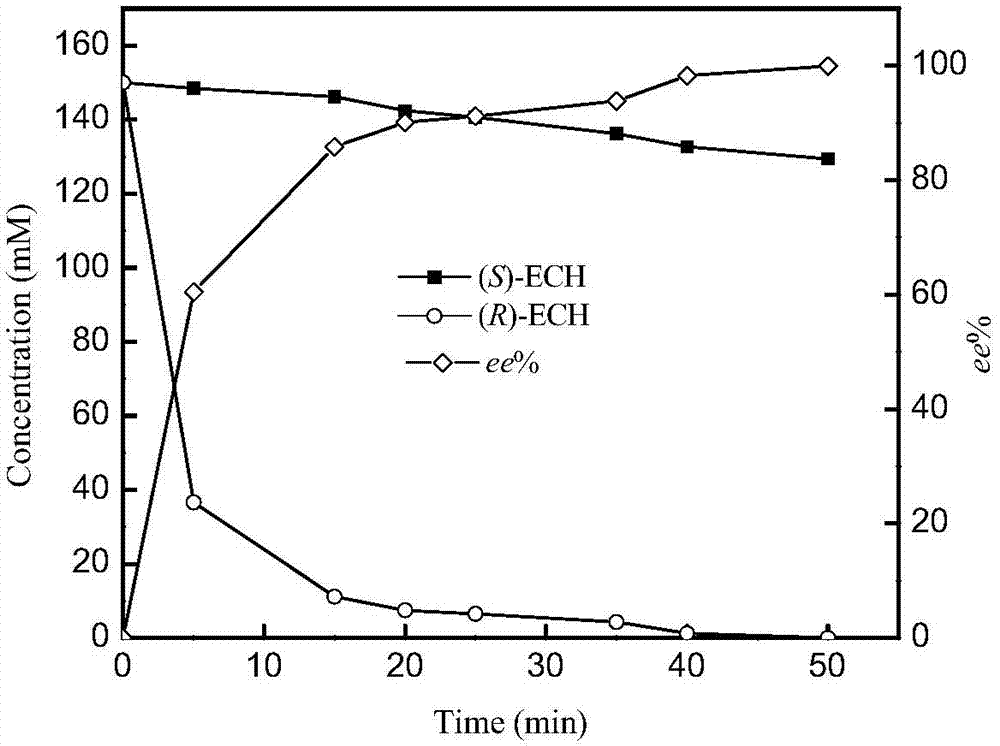
[0045] Activity, catalytic constant and stereoselective determination of embodiment 3 epoxide hydrolase mutants
[0046] The substrate and product in the reaction mixture are separated by gas chromatography, the decrease of substrate concentration in the reaction system before and after the reaction is measured, and the catalytic activity of the epoxide hydrolase is determined.
[0047] Gas chromatography GC-14C column type: BGB-175 capillary column; chromatographic conditions: column temperature 90°C, injection chamber temperature 220°C, FID detector 220°C, helium flow rate 1.6mL / min; split ratio 40: 1.
[0048] Enzyme activity unit (U) is defined as: the amount of enzyme required to catalyze 1 μmol epichlorohydrin within 1 min at 30°C and pH 8.0 is defined as 1U. Specific enzyme activity is defined as the number of units of activity per milligram of protein.
[0049] Epoxyhydrolase activity assay method is: total reaction system is 0.4ml, wherein contains 50mM substrate, p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com