Recombinant human hyaluronidase, preparation method thereof and adopted polyethylene glycol covalently modified compound and method
A technology of human hyaluronidase and polyethylene glycol, applied in the field of biomedicine, can solve problems such as prolongation of half-life, and achieve the effects of low production cost, high stability and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Embodiment 1, the preparation of recombinant human hyaluronidase
[0098] The preparation method of recombinant human hyaluronidase comprises the following steps: (1), the acquisition of human hyaluronidase gene; (2) the construction and transformation of expression vector Pichia engineering bacteria: the synthetic human hyaluronidase The cDNA fragment is inserted into the restriction site of the expression vector, and then directly transformed into the Pichia host bacterium; (3), screening, cultivation, and induced expression of positive clones: the positive clones are selected and cultured on the medium, and induced Target protein expression; (4), separation and purification of fusion protein: collect the Pichia pastoris fermentation broth, centrifuge to obtain the supernatant of the fermentation broth, and then purify the supernatant of the fermentation broth by chromatography; (5) crudely purified The protein was further separated and purified.
[0099]The recombin...
Embodiment 2
[0105] Embodiment two, the activity determination of recombinant human hyaluronidase
[0106] The amount of reducing sugar produced by hydrolysis of hyaluronic acid was measured by 3,5-dinitrosalicylic acid colorimetric method. The reaction system is 1 mL, and 2 mg / mL of hyaluronic acid is prepared with pH 5.5, 50 mM citric acid-disodium hydrogen phosphate buffer solution, and 900 μL of hyaluronic acid solution and 100 μL of the test sample are added to the reaction system. React at 38°C for 20 minutes, immediately boil in water for 5 minutes to terminate the reaction, centrifuge to remove the denatured protein precipitate; use the DNS method to measure the equivalent of reducing sugar produced (equivalent to the reducing power of an equivalent amount of glucose), and calculate the activity of the test product. The specific activity of the enzyme was calculated from the protein concentration. The specific activity of the purified human hyaluronidase is greater than 100,000 U / ...
Embodiment 3
[0107] Embodiment three, different molecular weight polyethylene glycols modify human hyaluronidase
[0108] 1. Preparation of modified products
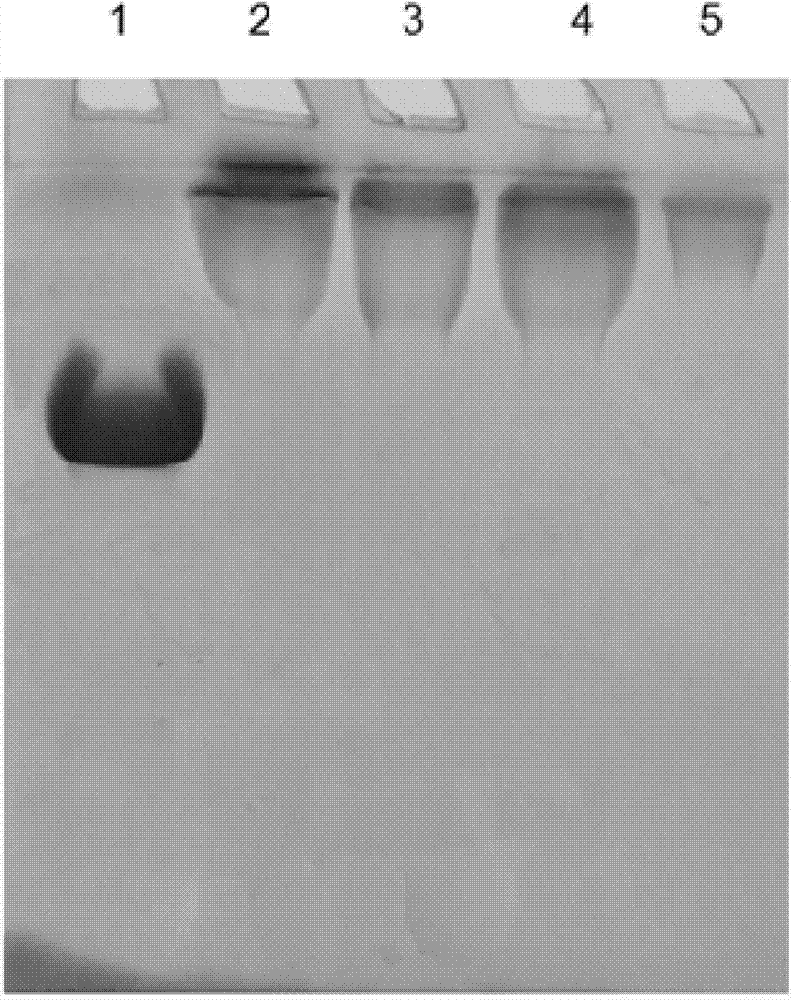
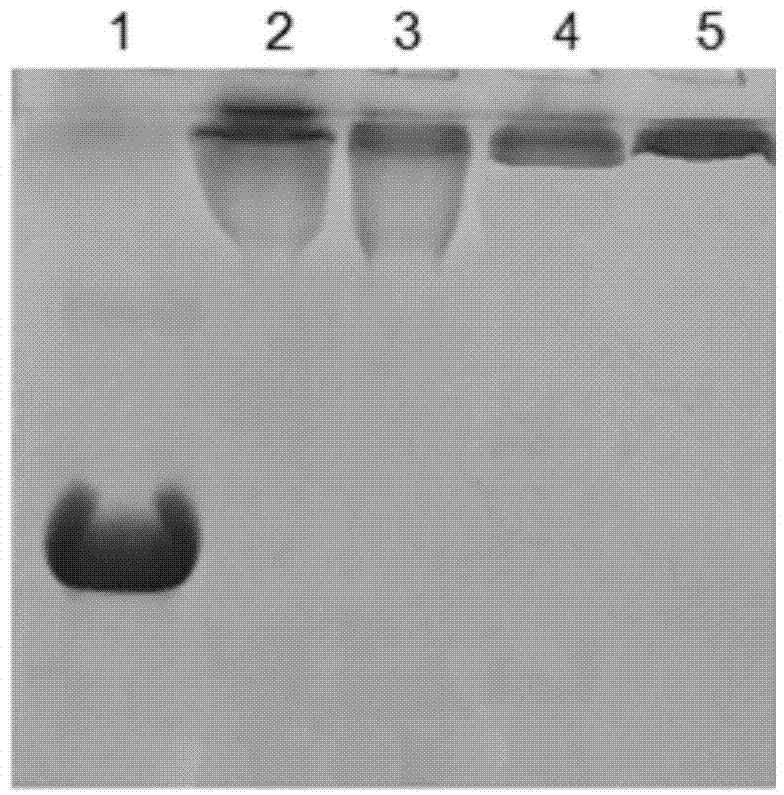
[0109] Purified Pichia pastoris expresses human hyaluronidase with a purity greater than 95%, a concentration of 2.0 mg / ml, using a G-25 column to exchange the buffer, and preparing an equilibrium buffer of 0.1 mol / L Na 2 PO 4 , 20mmol / L NaCl, flow rate 10ml / min, load the sample after the column effluent reaches equilibrium, and collect the protein peak. Monomethoxy PEG propionate with different molecular weights was added to the hyaluronidase with a different buffer system at a mass ratio of 10:1, and the reaction was terminated after stirring at room temperature for 2 hours for modification. Use a 50KD ultrafiltration membrane to ultrafilter the hyaluronidase PEG reaction mixture against 20mmo / L PB, pH 7.0 buffer solution to remove free PEG, which is the hyaluronidase PEG modified stock solution.
[0110] SDS-PAGE analysis was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com