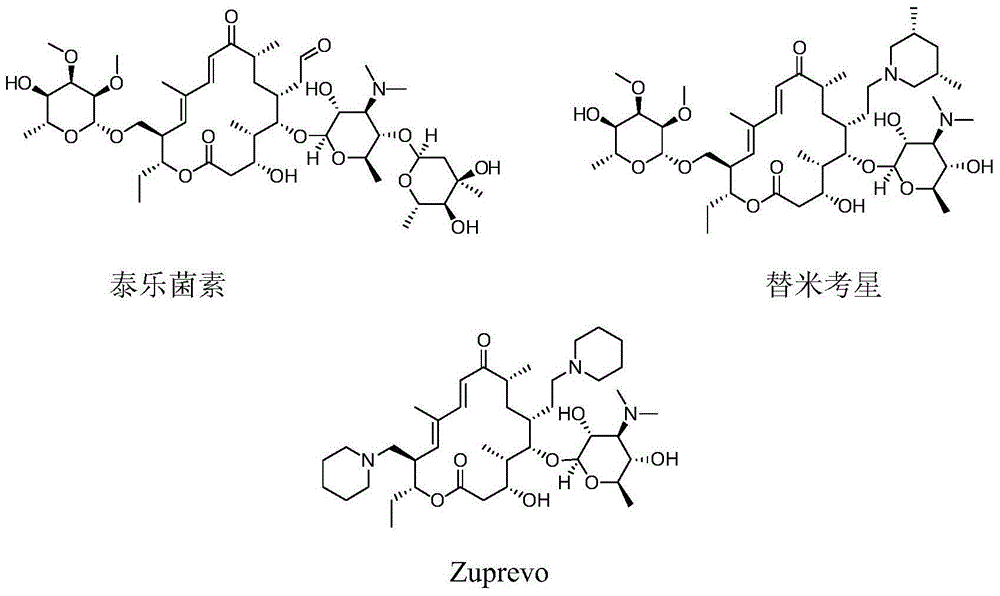
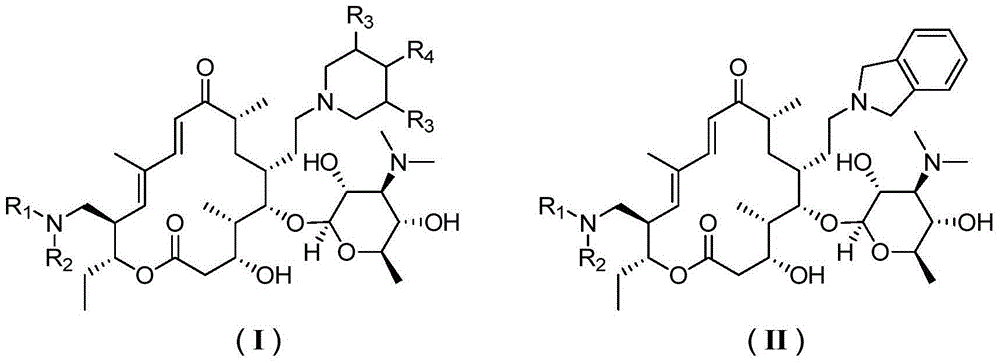
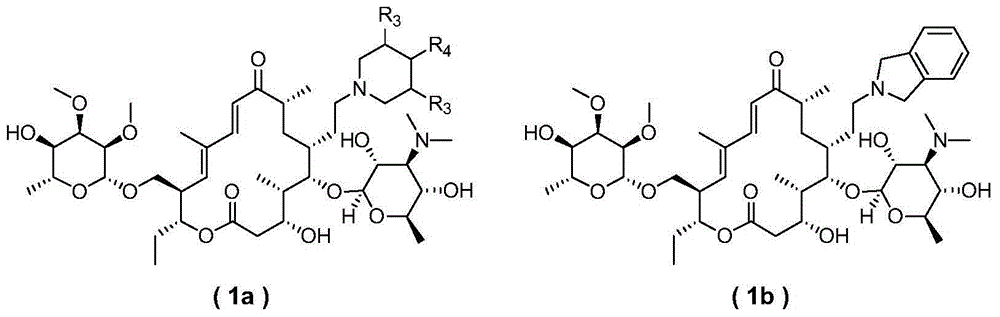
Novel sixteen-membered ring triamine lactone derivative and application thereof
A technology of sixteen rings, compounds, applied in the field of new antibiotics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1. (4R,5S,6S,7R,9R,11E,13E,15R,16R)-6-(((2R,3R,4S,5S,6R)-4-(dimethylamino)-3 ,5-Dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-16-ethyl-4-hydroxyl-15-((((2R,3R,4R,5R,6R) -5-Hydroxy-3,4-dimethoxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)methyl)-5,9,13-trimethyl-7-(2 -(piperidin-1-yl)ethyl)oxahexadecano-11,13-diene-2,10-dione (compound 1)
[0077] Tylosin (9.2 g, 0.01 mol), hexahydropyridine (2.55 g, 0.03 mol), and toluene (30 ml) were added together into a three-neck flask, stirred and heated to 75°C, and formic acid (2.5 g, 0.05 mol) was slowly added dropwise ), the temperature is controlled at about 75°C, the formic acid is dropped, and the reaction is carried out at 75-80°C for 2 hours. After the reaction is over, add 15% dilute hydrochloric acid to dissolve the viscous substance in the lower layer, and separate the water layer. The benzene layer was washed twice with 90 ml of dilute hydrochloric acid, and the aqueous layers were combined. Neutralize with 30%...
Embodiment 2
[0078] Example 2. (4R,5S,6S,7R,9R,11E,13E,15R,16R)-6-(((2R,3R,4S,5S,6R)-4-(dimethylamino)-3 ,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-16-ethyl-4-hydroxy-15-(hydroxymethyl)-5,9,13-trimethyl yl-7-(2-(piperidin-1-yl)ethyl)oxahexadecano-11,13-diene-2,10-dione (compound 2)
[0079] Compound 1 (9.0 g, 0.01 mol) was added into a three-necked flask, and then 30 ml of hydrobromic acid and 30 ml of water were added, and reacted at 57°C for 5 hours. Cool and filter, and the filtrate is neutralized with 30%-40% sodium hydroxide solution to a pH of about 10, and the precipitated product is solid and can be filtered. If the precipitated product is viscous, dehydration is required, and 4.7 grams of crude product is obtained by drying, and the productive rate is 70%. Theoretical value of mass spectrum: 666.45, measured value: 667.40 (M+H + ).
Embodiment 3
[0080] Example 3. (4R,5S,6S,7R,9R,11E,13E,15S,16R)-6-(((2R,3R,4S,5S,6R)-4-(dimethylamino)-3 ,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-16-ethyl-4-hydroxy-15-(iodomethyl)-5,9,13-trimethyl yl-7-(2-(piperidin-1-yl)ethyl)oxahexadecano-11,13-diene-2,10-dione (compound 3)
[0081] Add compound 2 (12.4 grams, 0.02 moles) dichloromethane (40 milliliters), triphenylphosphine (8 grams, 0.03 moles), pyridine (2 grams, 0.022 moles) in the there-necked flask, ice bath, make the temperature below 13°C. Iodine (8 g, 0.03 mol) was added in portions, and the temperature was controlled at 15±3°C after the addition of iodine, and stirred for 3 hours. Cool and filter, extract the filtrate twice with 30 ml of 15% dilute hydrochloric acid, combine the extracts, neutralize with 30% sodium hydroxide solution until the pH is 9-10, precipitate off-white solid product, and filter out the product. The product is dissolved in a small amount of dichloromethane, dried and concentrated, and petrol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com