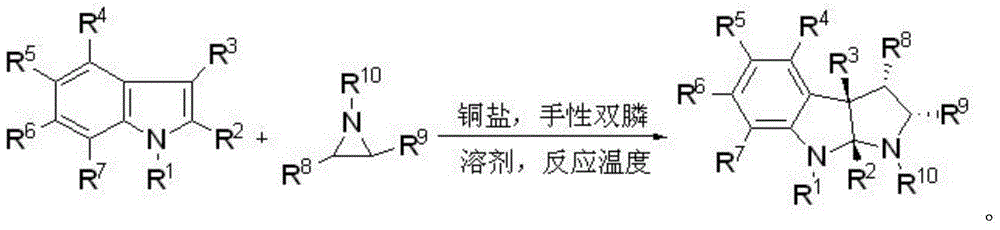
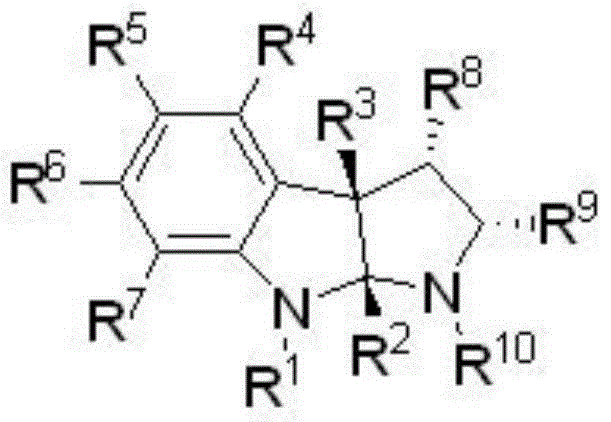
Preparation method of chiral hexahydropyrroloindole compound
A technology of hexahydropyrrole and indole, applied in the direction of organic chemistry, etc., to achieve the effect of less reaction steps, high yield and stereoselectivity, and easy availability of reaction raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Under argon atmosphere, add copper tetraacetonitrile boron tetrafluoride (0.0025mmol), (R)-XylBINAP (0.0015mmol) and dry m-xylene (0.25mL) successively into a dry Schlenk tube, and stir at room temperature for 20 minutes Then add 1,3-dimethylindole (0.05mmol dissolved in 0.25mL dry m-xylene) and 2-phenyl-N-p-toluenesulfonyl aziridine (0.11mmol) successively, and then stir at 15°C The reaction was carried out for 12 hours (monitored by TLC). After the reaction, a saturated aqueous sodium bicarbonate solution (5 mL) was added to terminate the reaction, extracted with ethyl acetate (5 mL×3), dried over anhydrous magnesium sulfate, and the solvent was removed under reduced pressure to obtain a crude product, which was passed through 1 According to H NMR analysis, the ratio of diastereoisomers dr>20:1, using petroleum ether: ethyl acetate = 20:1 mixed solvent (containing 0.5v% triethylamine) as developing solvent, separated by column chromatography Product (20.3 mg, yield: ...
Embodiment 2
[0035] Under argon atmosphere, add copper tetraacetonitrile boron tetrafluoride (0.0025mmol), (R)-XylBINAP (0.0015mmol) and dry m-xylene (0.25mL) successively into a dry Schlenk tube, and stir at room temperature for 20 minutes Then, 1,3-dimethylindole (0.05mmol dissolved in 0.25mL dry m-xylene) and 2-(4-fluorophenyl)-N-p-toluenesulfonyl aziridine (0.11mmol) were added successively to complete Then the reaction was stirred at 15°C for 18 hours (monitored by TLC). After the reaction, a saturated aqueous sodium bicarbonate solution (5 mL) was added to terminate the reaction, extracted with ethyl acetate (5 mL×3), dried over anhydrous magnesium sulfate, and the solvent was removed under reduced pressure to obtain a crude product, which was passed through 1 According to H NMR analysis, the ratio of diastereoisomers dr>20:1, using petroleum ether: ethyl acetate = 20:1 mixed solvent (containing 0.5v% triethylamine) as developing solvent, separated by column chromatography Product (...
Embodiment 3
[0039] Under argon atmosphere, add copper tetraacetonitrile boron tetrafluoride (0.0025mmol), (R)-XylBINAP (0.0015mmol) and dry m-xylene (0.25mL) successively into a dry Schlenk tube, and stir at room temperature for 20 minutes Then, 1,3-dimethylindole (0.05mmol dissolved in 0.25mL dry m-xylene) and 2-(4-chlorophenyl)-N-p-toluenesulfonyl aziridine (0.11mmol) were added successively to complete Then the reaction was stirred at 15°C for 32 hours (monitored by TLC). After the reaction, a saturated aqueous sodium bicarbonate solution (5 mL) was added to terminate the reaction, extracted with ethyl acetate (5 mL×3), dried over anhydrous magnesium sulfate, and the solvent was removed under reduced pressure to obtain a crude product, which was passed through 1 According to H NMR analysis, the ratio of diastereoisomers dr>20:1, using petroleum ether: ethyl acetate = 20:1 mixed solvent (containing 0.5v% triethylamine) as developing solvent, separated by column chromatography Product (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com