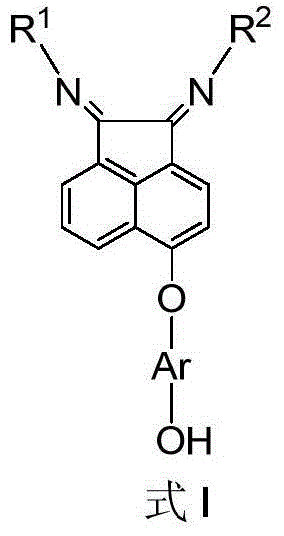
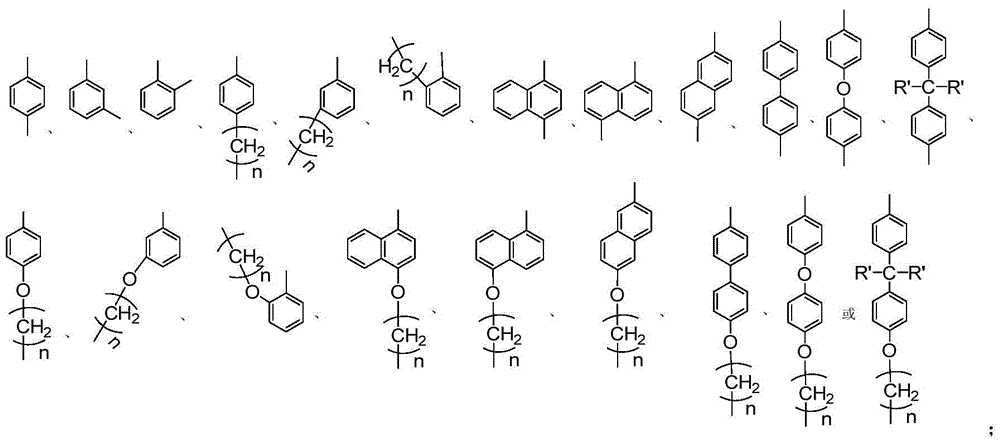
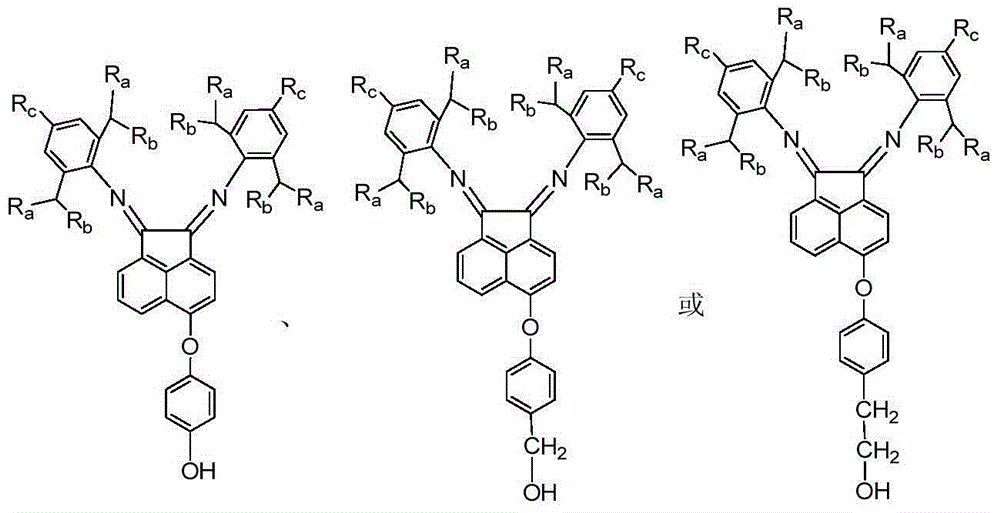
Supported alpha-diimine metal complex, and its application in olefin polymerization
A technology of metal complexes and diimine compounds, which is applied in the application field of supported α-diimine metal complexes in olefin polymerization, which can solve the problems of large amount of co-catalyst, difficult control of polymer morphology, poor thermal stability, etc. problem, to achieve the effect of simple and effective loading method, good particle shape and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 5-[4-(2-Hydroxyethyl)phenoxy]acenaphthenequinone bis(2,6-diisopropyl)phenylimine c1 (that is, the structure of compound C, where Ar=4-ethylphenyl , R 1 = R 2 = (2,6-diisopropyl) phenyl) synthesis:
[0042] Its preparation route is as follows:
[0043]
[0044] Synthesis of 5-[4-(2-hydroxyethyl)phenoxy]acenaphthenequinone b1 (that is, the structure of compound B, wherein Ar=4-ethylphenyl):
[0045] Add 13.1g (50mmol) of 5-bromoacenaphthylquinone in the reaction bottle of 100mL, 20.7g (150mmol) K 2 CO 3 , 50 mL of dry DMF, and start stirring. Add 13.8g (100mmol) a1 during the stirring process, react at 60°C, the reaction is stopped after the chromatographic trace traces that the reactants have completely reacted. The reaction dark brown solution was poured into ice water, and the obtained solid was purified by silica gel chromatography to obtain 15 g of yellow crystals of compound b1 with a yield of 94%. 1 H NMR (400MHz, CDCl 3 ): δ8.62(d, J=8.4Hz, 1H), δ8.16(d...
Embodiment 2
[0049] 5-(4-Hydroxymethylphenoxy) acenaphthoquinone bis (2,6-diisopropyl) phenylimine c2 (that is, the structure of compound C, where Ar = 4-methylphenyl, R 1 = R 2 = (2,6-diisopropyl) phenyl) synthesis:
[0050] Its preparation route is as follows:
[0051]
[0052] Synthesis of 5-(4-hydroxymethylphenoxy)acenaphthenequinone b2 (i.e., the structure of compound B, wherein Ar=4-methylphenyl):
[0053] Add 13.1g (50mmol) of 5-bromoacenaphthenequinone in the reaction bottle of 100mL, 7.45g (60mmol) p-hydroxybenzyl alcohol, 31.84g (150mmol) K 3 PO 4 , 0.47g (2.5mmol) CuI, 0.62g (5mmol) 2-pyridinecarboxylic acid and 50mL dry DMF, heated and stirred at 90°C for 24 hours. Then the dark brown solution of the reaction was poured into a saturated NaCl solution to become a brown suspension, extracted 2-3 times with dichloromethane, and the organic phase was washed with anhydrous MgSO 4 After drying, the solvent was evaporated under reduced pressure to obtain 13 g of brown crystals...
Embodiment 3
[0056] 5-(4-hydroxyphenoxy)acenaphthenequinone bis(2,6-diisopropyl)phenylimine c3 (i.e., the structure of compound C, where Ar=4-phenyl, R 1 = R 2 = (2,6-diisopropyl) phenyl) synthesis:
[0057] Its preparation route is as follows:
[0058]
[0059] The preparation process of compound b3 is the same as that of compound b1 in Example 1, wherein 5-nitroacenaphthenequinone is used instead of 5-bromoacenaphthenequinone in Example 1; compound a3 is used instead of a1 in Example 1. The preparation process of compound c3 is the same as that of compound c1 in Example 1, wherein compound b3 replaces b1 in Example 1, and the yield of compound c3 is 76%. 1 H NMR (400MHz, CDCl 3 )δ8.24(d, J=8.3Hz, 1H), δ7.37(t, J=7.5Hz, 1H), δ7.29-7.14(m, 8H), δ6.96(d, J=8.9Hz ,2H),δ6.86(d,J=8.9Hz,2H),δ6.65(d,J=7.1Hz,1H),δ3.09-2.98(m,4H),δ1.24-1.21(m ,12H),δ0.99-0.96(m,12H).MS(ESI):m / z 610(M+H + ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com